Chemistry of combustion processes. Explosion energy
Explosion energy
The main advantages of explosives over other sources of energy are compactness, transportability and the fact that the release of energy can take place in short times, making it possible to develop enormous power. So, with the explosion of a spherical charge weighing 1 kg and a density of 1.65 g / cm3, which is made of powerful explosive - hexogen, excited (initiated) in the center, the propagation velocity of the explosive transformation through the substance (detonation) is 8300 m / s Way r (radius of charge), which will pass the detonation, is determined from the equality
where Gup - mass and charge density, respectively.
The process implementation time is τ = r / D (D - detonation velocity).
Power N (kJ / e), developed during the explosion, can be estimated, knowing the amount of heat released q with the explosive transformation of 1 kg of RDX:
For RDX q = 5420 kJ / kg. The calculated power is overestimated, since the time during which the expanding gases perform work was not taken into account. It should be noted that it exceeds the capacity of the largest power plants in the world. Explosives capable of such rapid transformation are called blasting.
The blasting explosives emit initiating explosives, which have practically no independent use and are used only for the preparation of explosives. Means of blasting (blasting caps, explosive cartridges, fuses, detonating cords, etc.) are combined into a special subgroup. Blasting explosives, gunpowder and explosives make up a group of explosive materials. Both during detonation and combustion during firing, additional conditions are imposed on the conversion of explosives into gases: for gunpowers - steady-state combustion at different pressures, for blasting explosives - an extremely high rate of propagation of the transformation by substance.
However, explosives are capable not only of rapid transformations. If you ignite a small amount of explosives with conventional means and allow it to burn under atmospheric conditions without obstructing the discharge of gases, the combustion will take place slowly and calmly. The method of destruction of unserviceable explosives by incineration is based on this, with qualified execution, it is safe and convenient. In case of improper combustion, circumstances may arise under which combustion will spontaneously turn into detonation with faster conversion of explosives into gases.
What is an explosion? Explosion refers to the physical or chemical transformation of a substance, in which its energy quickly passes into the energy of compression and movement of the substance itself or the products of its transformation and the environment. The energy of the explosion may be different. The release of chemical, electrical, nuclear, thermonuclear, thermal, kinetic energy, elastic compression energy can be accompanied by explosive processes. For example, in the electric-spark method of destruction of materials, microexplosions are used, the source of energy of which is an electrical discharge, and the carrier of energy is the products of evaporation and decomposition or simply heating the medium in which the discharge is carried out. The explosion caused by the destruction of compressed gas cylinders, steam boilers, high pressure vessels, can also proceed at high speed and cause serious damage to the surrounding space.
However, the main importance is the use of potential chemical energy, which in many substances under certain conditions (as a result of a chemical reaction) can quickly pass into the energy of compressed gases. Substances capable of such transformations are called explosive, and the explosion - chemical. In the future, under the explosion, without special reservations, we will understand only the chemical explosion, and all the processes under consideration are attributed to the processes occurring during a chemical explosion.
An explosion can be characterized by the amount of energy released. Since this process is sometimes set by the parameters of the equipment (for example, for pneumatic radiators — by the chamber volume and the pressure of compressed air), one must be able to determine its energy. For a pneumatic radiator, it is equal to PV /(k - 1), where R - pressure of compressed gas; V - chamber volume; k - correction factor (for air k = 1.4), for electric-discharge radiator - UC2/2, Where WITH - capacity U - voltage. The energy of chemical explosives is most often set by the heat of explosion in kilojoules per kilogram. Naturally, the comparison of sources involves the transfer of energy from one unit to another (calories to joules, etc.).
In connection with the consideration of the explosion, let us touch upon the process, to a certain extent opposite to it, the impulsion, which is beginning to be applied in technology. With the explosion of the explosive charge, the energy level allows gases due to the expansion to perform work on the external environment. At impulsion the substance of the environment surrounding the source has a higher pressure than at the source itself, and when removing the separating obstacle it is possible to rush inside the source. Such a process can cause wave disturbances. The simplest example of an impulsion is a bulb of an electric bulb (it is evacuated), broken in air. The higher the pressure of the medium, the more energy can be released when the cavity collapses. In deep wells, it becomes significant even in small cavities. A similar phenomenon is observed during an underwater explosion, when the over-expanded explosion products collapse under the action of hydrostatic pressure. In seismic, this is recorded as the second impact of an explosion produced in a reservoir, with a sufficient depth of charge in it.
Chemical explosion - self-propagating chemical transformation of a substance that proceeds at high speed, heat generation and the formation of gases compressed to high pressure. Detonation is a particular case of an explosion carried out with a constant, maximum speed for a given substance.
First of all, it is necessary to estimate the energy (amount of heat) released during the explosion. Reactions are of two types - with the release of heat (exothermic) and with absorption (endothermic). The heat of formation of molecules — compounds from atoms (the heat of formation of the latter is zero) —can be either negative (it is necessary to spend additional energy on their formation from the elements) as well as positive. Heat release from explosives is usually caused by the reaction between the combustible components and the oxidizing agent (oxygen), which is part of it. If explosive is an individual chemical compound, then they will be different groups in the molecule, if mixed, they are different substances that make up the mixture. Their ratio determines the oxygen balance of the substance. When oxygen is not enough to completely oxidize the combustible component of the explosive, the balance is negative. In substances with a positive oxygen balance, part of the oxygen in the explosion remains unused and is unproductively lost. The properties of composite explosives can be changed by selecting the appropriate oxidants and flammable.
Oxygen balance is defined as the deficiency or excess (in grams) of oxygen required for oxidation or 100 g of explosive remaining during oxidation. For tetranitrometa C (ΝO2) 4 it is equal to +49, ammonium nitrate ΝH4ΝΟ3 - +20, trotyl C7H5N3O6 - -74, hexogen (СH2N2) 3 - -21.6. The maximum negative oxygen balance, by definition, is for hydrogen (-794), the maximum positive is for oxygen (+100).
An example of explosives with a negative oxygen balance is trotyl, a common high-explosive. Its chemical name is trinitrotoluene; the names tol, TNT are found. As can be seen from the structural formula, the combustible components — the atoms of hydrogen and carbon and the oxidizing agent — are oxygen, which is part of the nitro group (NO2), in the TNT molecule are not yet interconnected:
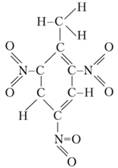
Based on the formula of the substance, you can specify in which direction the reaction will proceed in it, what products can be formed in this case. For TNT, the reaction of explosive decomposition is as follows:
The composition of the products depends on the explosion conditions and their final state (degree of expansion). Many explosive systems are the first to realize the reactions that are characterized by the maximum release of energy. Thus, in a substance having the formula of the form CaH4McOd, with a relatively small lack of oxygen [ d< (2a + b / 2)] first of all, hydrogen is reacted with oxygen as the most advantageous, since 255 kJ is released per unit of oxygen during the formation of H2O, and 187 kJ / during the formation of CO2 (the heat of formation of CO2 is 379 kJ / mol).
The approach to calculating the heat of transformation of explosives, in which those of possible reaction products are recorded, the formation of which ensures its maximum thermal effect, is called the principle of maximum operation. The calculation to some extent idealizes the process and gives the greatest accuracy for systems with a positive or with a slightly negative oxygen balance. In fact, the composition of the explosion products (PT) usually does not correspond to this calculation. The latter is due to the fact that the interaction continues for some time after the explosion and an equilibrium is established between the products of reactions with both positive and negative thermal effects. Examples of the latter are reactions
For approximate calculations of systems with a negative oxygen balance, you can use the Le Chatelier method, based on the principle of maximum volume, and if the volumes are the same, then the reaction with a large heat release has priority. The method is most valid for determining the final state of the explosion products. The predominant reaction is the oxidation of carbon to CO. If oxygen is underexposed, then it is equally spent on additional oxidation of CO and H2. The reaction of decomposition of RDX in this case is written as:
A more accurate calculation of the composition of the explosion products is performed taking into account the kinetics and conditions of the reactions. The accuracy of these calculations is not always sufficient due to the lack of reliable information on the state of the substance at high temperatures and pressures. The data on the heat and on the composition of the explosion products are also obtained experimentally, for which special methods of investigation of the explosion have been developed and successfully used.
As already noted, the explosion energy is characterized by the heat of explosive transformation of explosives. Knowing the heat of formation of precursors and products of the explosion and using the Hess law (the thermal effect of the process does not depend on the transformation path, but on the initial and final states of the substances), we can calculate the heat and a number of other parameters of the explosion. Such calculations can also occur in the practice of a geophysical engineer, since mixed explosives and gunpowder are used and will be used in increasing quantities.
Perform the calculation of a cheap explosive mixture of Idanit type from granulated ammonium nitrate with liquid fuel. For ease of calculation, instead of the petroleum products used - solar oil or kerosene, which are a mixture of substances - take benzene (C6H6). Consider the case when the explosive mixture is specified by the indication of the components and the oxygen balance. Let it be given that a mixture of benzene and ammonium nitrate should have an oxygen balance of -10, otherwise it will lack 10 g of oxygen to fully oxidize 100 g of the mixture. To begin with, we will determine what composition the mixture should have in order to satisfy the task.
In order to simplify the solution, let us imagine that our explosives, as it were, consist of two parts - one of the components (in this case, fuel, because the balance is negative), taken in an amount that provides the desired balance, and a mixture of specified substances of zero oxygen balance (sometimes called stoichiometric). If we add such a mixture to the calculated amount of fuel, bringing the total weight to 100 g, we will get a composition with a given oxygen balance.
Consistently carry out calculations.
On the oxidation of the benzene molecule (its molecular weight is 78)
will need 15 oxygen atoms. Accordingly, the amount of benzene x1, the oxidation of which requires 10 g of oxygen, we obtain from the proportion "benzene - oxygen is required":
![]()
Add to this quantity (100 - x1) g mixture of zero oxygen balance, we obtain a mixture of a given composition.
Let us turn to the calculation of the content of components in 96.75 g of a stoichiometric mixture. Determine the excess oxygen in the oxidizer. Saltpeter decomposes and releases oxygen:
moreover, 80 g of the oxidizing agent (molecular weight of ammonium nitrate 80) will release 16 g of oxygen. The reaction between benzene and ammonium nitrate in a mixture of zero oxygen balance is written as
Then we get the proportion: ![]()
Therefore, in 96.75 g of the mixture will be 5.85 g of benzene and 90.85 g of ammonium nitrate. Thus, the final composition of the mixture with a given oxygen balance and the composition of the explosion products, calculated according to the principle of maximum operation, will be:
Without resorting to the calculation, it was possible to immediately record the content of free carbon in the explosion products, since it is precisely its presence that will determine the negative oxygen balance of the mixture. Since the full oxidation of 12 g of carbon requires 32 g of oxygen, 10 g of oxygen can oxidize g of carbon, or a fraction of a mole of carbon. These considerations are good to use to verify the correctness of the calculations made.
To prepare such a mixture is very simple: the right amount of nitrate must be mixed with benzene. Having the initial components and knowing the composition of the explosion products, it is easy to calculate the heat of explosion of the system. The heat of formation of the initial components and products of the explosion is found in the relevant directories. So, for 1 mole of benzene it is (in kJ / mol) -39.1, ammonium nitrate - +410.8, CO2 - +444.2, H2O - +271.7.
Thermal effect of the explosion Qx charge in the problem can be determined from the equation
 (4.29)
(4.29)
It will be 374 kJ / 100 g of the mixture, or 3740 kJ / kg.
The volume of gaseous products formed (in liters) can be calculated from the reaction equation by multiplying the number of moles of gas by 22.4 (the volume of a gram molecule). Naturally, this should take into account the state of the substance. So, carbon (sublimation temperature above 3700 ° C at normal atmospheric pressure) will not give a gas phase, water, of course, will be steam. The volume of explosion products, referred to normal atmospheric conditions, is called given volume. In the problem discussed above, it will be about 800 l / kg.
If the heat and composition of the explosion products are known, then their temperature Tsun can be determined from the relation Tup = = Q / Cvcr where WITHv cp is the average heat capacity of the explosion products (at a constant volume) for the interval Τ 0–Τ charge It is significant to note that heat capacity is a function of temperature.
The latter introduces some (purely technical) complications into the calculation, since the type of dependence is known. However, it is easier to use ready-made data on the heat content of gases at different temperatures (Table 4.1).
Table 4.1
Change in heat content (internal energy) of some gases (kJ / mol)
|
Temperature, K |
C (graphite) |
||||||
Given the temperature and knowing the composition of the products of the explosion, you can find their heat content and compare it with the heat of the explosion. The comparison will determine the nature of the error made when choosing a temperature. Repeating the operation, but with a different (corrected) temperature, you can use the approximation method to find the temperature of the explosion.
It should be noted that the energy released during the explosion is relatively small: the most powerful explosives have an explosion heat of 6500-6700 kJ / kg. With the explosion of 1 kg of TNT, about 4000 kJ of heat is released in the air, and when burning 1 kg of diesel oil - about 44,000 kJ. But the heat of combustion of 1 kg of a mixture of diesel oil with oxygen will be only 10,000 kJ, and 1 kg of a mixture of diesel oil and air - 2670 kJ. It was repeatedly emphasized that, in addition to the release of energy, an explosion is characterized by the formation of products that are in a gaseous state at those temperatures that are reached during an explosion.
Although the heat of the termite reaction
about 2.3 times higher than the heat of explosive decomposition of TNT, its combustion proceeds calmly; the resulting products, even at those temperatures to which they are heated, remain liquid. But one has only to wet the termite, as the picture changes: heat turns water into steam, and burning is accompanied by explosive processes. The explosive in the volume occupied by the charge, the explosion immediately formed compressed to enormous pressure gases - reaction products, ensuring the realization of the released energy in the form of work done by expanding gases.
When creating an explosive, we are naturally interested in the fact that with a hundred explosions, more energy is released and compressed gases are formed that are able to realize the energy in the right way. Sometimes these requirements conflict. Thus, the energy of explosive decomposition can be increased by selecting the appropriate combustible, in particular, the introduction of metals into the composition of the explosive, for example, aluminum (the heat of formation of A12O3 is 1599 kJ / mol). Aluminum oxidation products are solids. The addition (up to a certain limit) of aluminum to some explosive mixtures can increase the efficiency of the latter. There are explosives with increased heat of explosion, containing in its composition metals. As already noted, the composition of the products of the explosion depends not only on the explosive, but also on the conditions of the explosion: the method of initiation, the size and design of the charge and the shell, the conditions of the environment in which the explosion takes place. If the initial products remain constant and the composition of the explosion products changes, the thermal effect of the explosion will also change, therefore for some explosives different values of the explosion heats are given depending on the conditions in which it is carried out. As an example, in the table. 4.2 shows (according to R. Schmidt) the composition of the products of the explosion of TNT (density 1.52) when a charge explosion is excited by weak and strong initiators.
In order for the transformation of explosives due to the onset of a rapid chemical reaction to pass over the entire charge, the process must be self-propagating. To do this, the chemical reaction must have the appropriate kinetic characteristics, and the release of energy must compensate for the inevitable losses. The reaction rate, the ability to self-propagation, exothermicity, gas formation are closely interrelated and affect each other and the boundaries of the explosive process.
Table 4.2
The composition of the products of the explosion of TNT
In tab. 4.3 shows the characteristics of a number of explosives and powders used by industry.
Table 4.3
Explosive characteristics of some explosives
An explosive in an explosion releases energy due to the fact that a small amount of solid or liquid explosive turns into a huge amount of gases heated to temperatures of thousands of degrees. For different types of explosives, the volume of released gases per 1 kg of explosives, having an initial volume of not more than 0.8-1 l, is a value from 300 to 1000 l and more. Formed during the explosion of hot gaseous decomposition products of explosives begin to expand, producing mechanical work. Thus, explosives have a reserve of latent chemical energy released during an explosion. However, not only explosives have latent energy, but, for example, gasoline, coal, firewood and other combustible substances. This energy of flammable substances can be released during combustion. Why, for the purpose of destruction and throwing, explosives and gunpowder are used instead of, for example, gasoline? It is known that 1 kg of gasoline energy is 10 times more than 1 kg of TNT, and 12 times more than smokeless pyroxylin powder. But the explosive charge and the charge of gunpowder with enormous speed turn into gases, and gasoline or any fuel cannot burn without enough air or free oxygen. Combustion of 1 kg of gasoline requires as much oxygen as it is contained in 15.5 kg of air. Therefore, the heat of combustion (energy) of the fuel must be counted on for 1 kg of its mixture with the oxygen necessary for its complete combustion. With this calculation, the difference in the energy of combustion of a mixture of gasoline with oxygen and the energy of explosion of an explosive charge of the same amount is lower than that given above, however, in this case, the amount of energy released during the combustion of gasoline is more: smokeless powder - 2860 kJ / kg, trotyl - 4100 kJ / kg, a mixture of gasoline with oxygen - 11,000 kJ / kg. Consequently, it is not the amount of energy contained in explosives and gunpowder that is the main reason for their use for the purposes of destruction and throwing. The main reason is not in the magnitude of energy, but in its very rapid release. If combustion of 1 kg of gasoline in an automobile engine occurs (depending on engine power and load) in 10–60 min, 1 kg of powder burns in the charging chamber of an artillery gun for a few thousandths of a second, and the explosion of 1 kg of TNT lasts only 30 -40 hundredths of a second. Energy during an explosion is released tens of millions of times faster than during the combustion of fuels. This explains the enormous power of the explosion. However, it is more correct to calculate the power of the explosion not by the detonation time of the entire charge, but by the time the explosion products exceed the normal atmospheric pressure level, the achievement of such a level as a result of high-speed shooting of the explosive process occurs within a few milliseconds. In this case, the power of 1 kg of TNT is expressed as more than 1 MW. But even in real conditions, this power cannot be fully realized because of its short duration, the mass inertia of the material being transferred or destroyed, on which it acts, as well as the losses due to environmental heating, excessive grinding and spreading of it, to residual heat in explosion products after their final expansion and on the inevitable chemical losses. As a result, useful mechanical work often does not exceed 1-2%, and when exploding in a solid medium - 8-9% of the energy contained in the explosive. However, the huge amount of potential energy contained in explosives and gunpowder makes them indispensable, despite its incomplete use in an explosion. High power is typical for explosives and when they are used for projectile throwing. The power of the powder charge of a large caliber artillery shot is 10 MW.
The first chronologically (the end of the nineteenth century) was the thermal theory, the founders of which are the painter, Le Chatelier and Nusselt. The basis of this theory is the van't Gough hypothesis about the temperature dependence of the rate of a chemical reaction. The condition for a thermal explosion is the predominance of heat input due to the reaction energy over the removal of heat into the environment. In this case, the system accumulates heat, which leads to self-heating and, accordingly, to self-acceleration of the reaction.
Occurrence in the combustible oxidation reaction system is most often associated with heating of the system by one or another source of ignition. When the fuel system is heated, the energy of the fuel molecules and oxygen increases and, when it reaches a certain value, they are activated, i.e. active centers (radicals and atoms) with free valences are formed, as a result of which the molecules of a combustible substance easily join with oxygen from the air. A.N. Bach and C. Engler in 1898 independently proposed the peroxidation theory of oxidation, according to which when a combustible system is heated, oxygen is activated by breaking one bond between atoms, and the active molecule enters into a compound with a combustible substance, without breaking down into atoms and forming peroxide compounds of the type: R 1 -OOR 2 or ROO-OH.
However, the peroxide theory is not able to explain some characteristic features of the oxidation process, for example, a sharp effect, sometimes insignificant traces of impurities.
The rate of chemical reaction, m / s, can be expressed on the basis of the Arrhenius law, by the following equation:
![]() , (1.12)
, (1.12)
where is the reaction rate constant (the rate of chemical reaction at concentrations of reagents reduced to unity);
The concentration of reagents, mol / m 3;
The stoichiometric coefficients determined by the ratio of the concentrations of the initial reagents in the stoichiometric reaction equation;
The basis of natural logarithms;
The universal gas constant, = 8.3 J / (mol ∙ K);
- temperature, K.
The thermal theory of self-ignition (also called the theory of thermal explosion) is based on a comparison of the rates of heat generation during exothermic oxidation and heat removal from the reacting mixture in the wall of the vessel containing it. The condition of self-ignition is determined by the equality of these speeds. The temperature of the vessel walls at which this equality is achieved is called the autoignition temperature. Starting from this temperature (characteristic in each case for the given specific conditions - the size and shape of the vessel, the thermophysical properties of the gas), self-heating occurs, which can lead to a flash (self-ignition).
Taking into account the above, for self-heating in a reactive environment we can write down:
where is the heat capacity at a constant volume, J / K;
Gas density, kg / m 3;
Gas temperature, K;
Time, s;
Reaction heat effect, W;
Reaction rate, m / s;
The surface of the reaction vessel, m 2;
The volume of the reaction vessel, m 3;
Heat transfer coefficient, W / (m 2 × K);
The temperature of the vessel wall, K.
DA Frank-Kamenetsky proposed a criterion for thermal ignition, based on a violation of the stationary temperature distribution in a jet vessel with a sufficient heat release rate:
 , (1.14)
, (1.14)
where is the characteristic size of the vessel, m;
Preexponential factor;
Thermal conductivity of the gas mixture, W / (m × K);
A dimensionless criterion of 3.3; 0.88 and 2, respectively, for spherical, plane-parallel and cylindrical vessels.
The criterion of D.A. Frank-Kamenetsky should be understood as follows: if during the substitution of all parameters that determine, we get a value, then there will be no ignition, when ignition occurs. From the equation of the criterion it follows that the heat of reaction and the radius of the vessel in which the reaction takes place are important determining factors of an explosion. The thermal explosion is expressed the brighter, the better the inequalities are fulfilled:
If these inequalities are fulfilled poorly, then the thermal explosion degenerates — simultaneously with the rise in temperature, a fast burnout of the original substance occurs, which smears the explosion picture.
The occurrence of combustion is most often associated with the heating of the combustible system by one or another source of ignition. According to the theory of academician N.N. Semenov, the oxidation process is accompanied by the release of heat and under certain conditions can accelerate. This process of self-acceleration of the oxidation reaction with its transition to combustion is called self-ignition.
In the case of thermal self-ignition, it occurs due to the excess of the heat release rate over the heat sink rate.
Consider the process of thermal ignition for example of combustible gas or vapors of a combustible liquid with air, placed in a vessel with a volume. With increasing temperature of the vessel and the combustible mixture, the reaction rate and heat will increase. The dependence of the rate of heat release on temperature, J / s is determined by:
![]() , (1.15)
, (1.15)
where is the heat of combustion of gas, J;
The volume of the combustible mixture, m 3;
Reaction rate constant;
Concentration of the reactant, kg / m 3;
Reaction order;
Activation energy, j / mol;
Universal gas constant J / (mol · K);
The temperature of the mixture, K.
The heat released is transferred to the combustible mixture, and it heats up. As soon as the temperature of the mixture exceeds the temperature of the vessel walls, heat removal through the vessel walls will begin per unit of time, proportional to the temperature difference between the mixture and the vessel walls and is determined by the relationship:
![]() , (1.16)
, (1.16)
where - the rate of heat removal through the walls of the vessel, j / s;
Heat transfer coefficient, J / (K · m 2 × s);
The surface of the vessel walls, m 2;
The temperature of the mixture, K;
Temperature of vessel walls, K
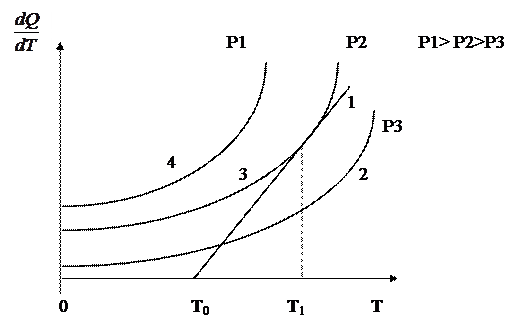
Figure 1.5 - The dependence of heat generation on temperature at different pressures
In Figure 1.5, curves 2, 3, and 4 show the dependence of heat generation on temperature at different pressures and the same mixture composition. At constant vessel and medium temperatures and constant mixture composition, the amount of heat removed from the combustion zone is characterized by a straight line 1. When the mixture composition changes, the rate of heat loss and, consequently, the slope of the straight line will change. The higher the pressure, the more heat is generated during the reaction (curve 4). Under conditions determined by curve 2, self-ignition cannot occur, since heat loss (straight line 1) is higher than heat generation at this pressure. The point of tangency of curve 3 with a straight line corresponds to the equilibrium between the heat released and removed at - the minimum auto-ignition temperature of a given combustible system under given conditions. With a small supply of energy from the outside, self-ignition is possible. Curve 4 describes the conditions under which self-ignition is unavoidable, since heat is released more than it is removed.
Analyzing the given scheme, N.N. Semyonov established dependence:
 , (1.17)
, (1.17)
where is the minimum ignition pressure, Pa;
The minimum temperature of self-ignition, K;
Reaction order;
Constant, depending on the composition and other properties of the mixture.
Based on this equation (1.17), one can theoretically determine in advance whether a self-ignition of a combustible mixture is possible under these specific conditions. The relationship between the minimum pressure and the autoignition temperature was confirmed by numerous experiments and proved valuable in studying combustion processes.
Chain Theory of Combustion
As early as 1928, N.N.Semenov advanced the idea of the possibility of the existence in chemical systems of two types of explosions — chain and thermal.
Chain reactions are those that go through a series of stages (through a series of intermediate reactions) in which intermediate compounds with free valencies are formed, the so-called active centers, which are the germs of the subsequent fast-flowing stages of the process.
The concept of a chain reaction first appeared in 1913, when a German physical chemist M. Bodenstein found that when a mixture of hydrogen with chlorine was illuminated, the chlorine molecule, absorbing a quantum of light energy, breaks up into atoms:
![]() .
.
Chlorine atoms instantly react with hydrogen, resulting in an explosion of the mixture. Activation of one molecule of chlorine would have caused the formation of two molecules:
![]() .
.
However, experiments show that this produces 100,000 molecules of hydrogen chloride. This can be explained if it is assumed that the interaction of chlorine with hydrogen produces a product that, when it enters into secondary reactions, is revived and can continue the reaction. This assumption corresponds to the following reaction scheme:
I Primary Reaction
IV open circuit
According to this scheme, the activation of one chlorine molecule (I) causes the appearance of two chlorine atoms - two active centers of the chain reaction. Each of the chlorine atoms gives rise to its own chain reaction, in which the active center is continuously restored (II, III). Thus, under the influence of the initiating reaction (I), successive reactions occur, forming a chain (II, III, etc.). The number of such reactions from the moment of chain initiation to its breaking is called the chain length. A chain can break at the collision of chlorine (IV) atoms or hydrogen (V) atoms and the formation of molecules from them, or at the collision of active centers with the surface of a solid. This is a typical non-branching chain reaction. In it, each active center causes the appearance of only one new active center, so the reaction can continue, but not be accelerated.
In a branching chain reaction, each active center gives rise to two or newer active centers.
According to the theory of chain reactions, the oxidation process begins with the activation of a combustible substance.
Practice has shown that ignition can occur under isothermal conditions, i.e. without raising the temperature of the reaction medium (“cold” ignition of the mixture). In this case, they speak of a chain (isometric) explosion.
Two initial components: fuel and oxidant, being in a relatively stable molecular state, before being associated with new, more stable combustion products, undergo a whole chain of complex intermediate transformations, which result in the formation of unstable products: atoms, radicals, excited molecules with relatively large the degree of ionization (formaldehyde, hydrocarbon and hydrochloride radicals, atomic oxygen and hydrogen).
Kondratyevu V.N. It was possible to detect in the flame of various hydrocarbons high concentrations of atomic oxygen (O), hydroxy acid radical (OH), hydrocarbon radicals (CH 3), carbon monoxide (CO), formaldehyde (CH 2 O) and others. The concentrations of these substances in the flame were thousands and millions of times greater than their equilibrium concentrations during thermal decomposition at flame temperature of the final reaction products, for example, H 2 O → H + OH.
The results of these observations led to the conclusion that the atoms and radicals under consideration appear in the reacting gas not due to the final decomposition of the products, but are intermediate products of the reaction.
Thus, the chain mechanism of ignition is based on a whole chain of chemical transformations of one substance into another, the result of which is the formation at certain intermediate stages of chemically very unstable products, called active centers, which easily react with each other and with the molecules of the initial substances to form New active centers and end products, for example, for methane-air mixture of H 2 O and CO 2.
The high reactivity of radicals and atoms is explained by the low activation energy of their reactions, which is close to the activation energy of atomic reactions:
OH + H 2 = H 2 O + H - 25 kJ / mol
СН 3 + С 3 Н 6 - 12.5 kJ / mol
CH 3 + C 6 H 6 - 23.5 kJ / mol
H 2 O → H + OH
Any of the obtained active particles (H or OH) being extremely unstable and, therefore, chemically active, colliding with the molecule of the original substance, splits, forming new active particles:
H + O 2 = OH + O
OH + H 2 = H 2 O + H
The resulting particles of the reaction of the active particles H and HE again enter into reactions, and the particles of O interact with hydrogen:
O + H 2 = OH + H.
That is, as a result of the reaction between the active particles and the molecules of the starting materials, not only the final products, but also new active particles are formed. The active particles formed as a result of the reaction give rise to new stages of chemical transformation, which will occur before the complete consumption of the starting materials.
Such repeatedly repeated chemical reactions are called chain reactions, and active particles, which give rise to new chains of transformations, are active centers.
The above chain process of hydrogen combustion can be represented as a diagram (Figure 1.6).

Figure 1.6 - Diagram of the chain burning of hydrogen
It can be seen from the diagram that only hydrogen (H) particles, which are active centers, give rise to new transformation chains. At the same time, in each link of the chain process, as a result of the reaction between the active center H and the oxygen molecule O 2, in addition to the final product H 2 O, 3 new active centers H are formed, giving rise to new transformation chains.
Such a chain reaction that occurs with an increase in the active centers is called branched. The reaction develops as an avalanche and proceeds at very high rates, far exceeding the speed of ordinary molecular reactions.
A typical reaction with a non-branching chain is the interaction of chlorine with hydrogen. The active centers of this reaction are alternating atoms of chlorine and hydrogen. When a chlorine atom reacts, one hydrogen atom is formed, just as when a hydrogen atom reacts, one chlorine atom is formed. Therefore, the reaction may continue, but not accelerated.
The well-known fact that the photochemical reaction of chlorine with hydrogen still ends in an explosion (self-ignition) is due to the fact that, at a sufficiently high chain reaction rate, the heat release exceeds the heat sink, as a result of which the mixture is very hot, and the conditions necessary for thermal self-ignition arise.
When a branched chain reaction occurs, which is typical for hydrocarbons, the concentration of active centers may increase regardless of the initial initiation conditions, and if the rate of formation of active centers during branching exceeds the rate of chain breakage, then a self-accelerating avalanche-like process occurs, resulting in ignition.
The theory of chain reactions allowed us to explain many features of the combustion processes (the strong influence of impurities, the limits of auto-ignition by pressure, catalysis and inhibition of pressure, etc.), which cannot be explained by thermal theory. The mechanism of the occurrence and development of real fires and explosions is characterized by a combined chain-thermal process. Beginning in a chain way, the oxidation reaction due to its exothermicity continues to be accelerated by the thermal path. Therefore, ultimately, the critical (limiting) conditions for the occurrence and development of combustion will be determined by heat generation and heat exchange conditions of the reacting system with the environment.
Many chemical processes are based on chemical chain reactions. Such processes include, for example, polymerization processes that form the basis of the production of synthetic rubbers, plastics, polymer fibers and many other products. They also include such important industrial processes as the production of synthetic fatty acids, replacing the previously used edible fats in the production of cleaning lubricants, cracking - the process of obtaining high-quality fuels from oil, etc.
Combustion - fast flowing chemical interaction of combustible substances with an oxidizing agent, accompanied by the release of a large amount of heat and a bright glow (flame). Combustion is possible only if there are three factors: a combustible substance, an oxidizing agent, a heat source.
Heat source (fire) can be an open flame, spark, heat, heated as a result of friction (belts in the belt transmission), impact, pressure. The heat source can also be electric (heating of conductors, arc), chemical and radiant energy of the sun.
Oxidizing agents are chlorine, fluorine, bromine. The most common oxidizing agent is air oxygen; the combustion process will depend on its content in the air. If oxygen in the air is more than 14-16%, then steady burning is observed. When the oxygen content is less than 14%, smoldering is observed, and when its content is less than 8-10%, smoldering also stops.
Combustible matter. They can be gases (ammonia, acetylene, hydrogen), liquids (gasoline, acetone, alcohol), solids (coal, wood). In order for a solid or liquid to ignite, it is necessary to heat them up to a temperature with the help of a heat source so that an intense release of combustible vapors occurs from their surfaces. When a certain concentration is reached, these vapors light up. The gases in the combustion process do not change their state of aggregation.
There are the following types of combustion: 1) flash; 2) ignition; 3) self-ignition; 4) spontaneous combustion; 5) smoldering; 6) the explosion.
1. Flash is called instantaneous combustion of a mixture of air oxygen with vapors, gases, dust, not accompanied by the formation of compressed gases. The flash point is the lowest temperature of a combustible substance, at which vapors or gases are formed above its surface, capable of flashing from the ignition source, but for the subsequent steady burning their rate of formation is insufficient.
Depending on the magnitude of this temperature, flammable liquids are divided into:
a) flammable (flammable liquids - gasoline, acetone, alcohol) - tf ≤ + 45 ° C;
b) combustible liquids (GZh - oils, diesel fuel, fuel oil) - t vec\u003e + 45 ° C.
2. Ignition called sustained continuous burning of a substance from a heat source. The minimum temperature of a combustible substance at which it ignites from an ignition source and continues to burn after its removal is called the ignition temperature. It is higher than the flash point.
Concentrations of flammable substances in the air, at which ignition or explosion is possible, are within certain limits: the lower - CWP and the upper - ERW. Inflammation of combustible mixtures is not possible at concentrations lower than a CEL (not enough combustible molecules in the mixture) and higher ERW (not enough oxygen molecules in the mixture). The greater the difference between ERW and CWP, the more dangerous the substance. The values of these parameters can decrease, for example, with an increase in the moisture content of the dust-air mixture (PLN), for example, a mixture of air with sugar, flour, coal dust.
Let us give examples of the values of NVP and SVV for a number of gases and vapors of liquids:
Acetylene 3.5-82%;
Natural gas 3.8-19.2%;
Gasoline 1-6%;
Carbon monoxide 12.8-75%.
3.Self ignition- the process of ignition of substances from an external source (flame, heated or heated body) without direct contact with it at the ignition temperature. This temperature will decrease with increasing pressure and for most flammable gases is in the range of 400-700 ° C, for wood - 340-400 ° C; coal - 400-500 ° C. An example of self-ignition: heating and subsequent ignition of wood, paper, located near an open flame (without contact with it) or hot objects (coals, open spiral of the heater).
4. Spontaneous combustion substances occur as a result of physical, chemical and biological reactions occurring in the substance (material) itself, leading to burning in the absence of an ignition source.
When stored in large quantities of wet grain, hay, straw, and insufficient ventilation within these materials, biochemical processes (decay) occur with the release of heat. The temperature of these materials increases, their large mass (ricks, stacks) prevent dispersal of the generated heat into the environment, which causes a fire. Such materials should be dried well before storage. Tissue contact (overalls, cleaning material) containing oil stains and folded in a heap without ventilation will also ignite spontaneously. Therefore, work clothes should be hung in such a way as to ensure free access of air, and oil should be promptly removed from work areas.
Depending on the reaction rate, the combustion process proceeds as corruption (speed a few cm / s), actually combustion (a few m / s) and explosion (several hundred and thousand m / s).
5. Explosion- a sudden change in the physical and chemical state of a substance under the influence of high temperature, pressure, chemical reagents. With the explosion, the volume of generated gases and vapors dramatically increases, a huge amount of energy is released, which in the form of a shock wave is capable of performing mechanical work (to destroy buildings, structures, injure people).
Combustion of materials may be complete or incomplete. During complete combustion (excess oxygen) non-combustible products are formed (CO 2 and H 2 O). In case of incomplete combustion (lack of O 2), products of incomplete oxidation (CO, alcohols, acids) are formed. They are toxic and explosive. Therefore, when organizing the process of burning fuel (in boilers, stoves), it is necessary to ensure a sufficient amount of oxygen in the furnace.
1. Basic concepts of the theory of combustion.
1. Features of combustion processes.
Combustion is a complex physicochemical process during which chemical transformation
schenie accompanied by the release of energy (mainly in the form of heat and radiation)
and heat and mass transfer with the environment.
The basis of the combustion process is a chemical reaction that can proceed with a
acceleration. The reasons for self-acceleration can be:
1. Heat accumulation in the system - thermal acceleration
2. Accumulation of active particles - chain self-acceleration
3. Autocatalysis is the acceleration of the reaction with its products.
In many cases, practically important combustion processes obey purely physical
due to the fact that at high temperatures chemical transformation can proceed at high speeds and the chemical process is subject to purely physical laws, such as heat transfer and diffusion, and is regulated by them. This means that a chemical reaction that can proceed with a high rate of
has a limited speed and obeys the laws of one or another
zic phenomenon.
The main feature of combustion processes is that the conditions of self-acceleration
chemical reaction created by itself. In cybernetics, this phenomenon is called positive feedback, that is, with a small change in external conditions, it is possible to switch from a stationary mode of reaction at a low speed to a mode, to o
where the reaction rate increases exponentially. Such phenomena of a sharp change in the mode of the reaction with a small change in external conditions are called
are critical phenomenaand the conditions under which they are observed are called
critical conditions.
Critical phenomena include:
1. Self ignition
2. Ignition
3. The concentration limits of flame propagation.
Critical phenomena occur not from the fact that the laws of nature drastically change, but
are a consequence of the imbalance between the reacting system and the environment
environment.
The condition of self-ignition is the impossibility of thermal or diffusion equal
environmental conditions, the ignition condition is an imbalance under the given initial conditions.
The second feature of combustion processes is their ability to spread
in space. In the thermal mode of combustion, the propagation occurs through heat transfer; in the case of chain or autocatalytic - through diffusion of active particles.
2. Types and modes of combustion.
1. According to the aggregate condition of the participants:
a. Combustion gas systems - homogeneous combustion
b. Combustion of solid and liquid combustible (solid-liquid-system and solid-gas systems) - heterogeneous combustion
c. Combustion of condensed systems (solid-liquid systems,
liquid - liquid, solid - liquid).
2. By the speed of propagation process:
a. Deflagration burning - slow propagation of the process (by heat conduction or diffusion)
b. Detonation burning - the rapid spread of the process (with
the power of the shock wave).
3. By aerodynamic conditions:
a. Laminar burning is a smooth flame front.
b. Turbulent combustion is a highly curved flame front.
Examples of various types of combustion on the aggregate state of the participants:
Homogeneous combustion:
Organic burning in oxygen
CH4 (g) + 2O2 (g) = CO2 (g) + 2H2 O (steam)
Combustion in the presence of other oxidizing gases
H2 (g) + Cl2 (g) = 2HCl (g)
Decomposition of unstable substances (ozone)
2O3 (g) = 3O2 (g)
Heterogeneous combustion:
Liquid hydrazine burning:
N2 H4 (g) + O2 (g) = N2 (g) + 2H2 O (steam)
Carbon burning:
C (TV) + O2 (g.) = CO2 (g.)
Decomposition of unstable substances (acetylene)
C2 H2 (g) = 2C (s.) + H2 (g)
Combustion systems burning:
KClO3 (sv.) + Al (sv.) = KCl (sv.) + Al2O3 (sv.) 2NH4 NO3 (sv.) = 2N2 (g) + 4H2 O (steam) + O2 (g)
3. Thermodynamics of combustion processes. Heat balance.
Combustion temperature: It is customary to characterize the combustion processes by four temperatures
peruraty burning
Theoretical | T Theor | Determined by the heat of stoichiometric combustion (i.e. |
||
the mixture corresponding to the reaction equation), taking into account its heating and dissociation |
||||
combustion products without heat exchange with the environment. |
||||
Calorimetric T calor - is determined by the heat of combustion of stoichiometric |
||||
mixture with an initial temperature T 0 273K without taking into account heat exchange with the
environment.
3. Adiabatic Tg ad - is determined by the heat of combustion of a mixture of arbitrary composition without taking into account heat exchange with the environment.
4. The actual Tg action is the actually observed (measured) temperature of
Ratio between different definitions of burning temperature
The heat balance of combustion processes is based on the determination of the heat absorbed
burning products. The heat balance equation has the form:
Q pq Q pQ ishQ pot, |
where Q PG is the heat absorbed by the combustion products, Q p is the heat of chemical reaction,
Q ex is heat received from external sources, Q sweat is heat loss.

burning. Almost the dissociation of combustion products makes a significant contribution only at temperatures above 20,000 C.
There are higher and lower calorific value. In the first case, water, as a combustion product, is taken in liquid form, in the second case, in vapor form. Since the number of you
the heat that is divided depends on the amount of the burned substance, the molar temperature is
raft and specific heat of combustion. Those. heat released during combustion 1
mole or kilogram of combustible material.
To calculate the specific heat of combustion (in kJ / kg), the D.I. formula is often used.
Mendeleev
Q p 339, 4C 1257H 108.9 O N S 25 9H W, |
where X is the content of the element in the composition of the fuel in% (mass.), W - humidity.
The general approach to calculating the heat of reaction is based on chemical thermodynamic
done by the system. Then from the first law of thermodynamics (the law of energy conservation
gii) follows | |||
where Q is the heat received by the system, U is the change in internal energy, W is the work, |
|||
made by the system. For infinitely small changes, we have | |||
dU is the total differential of the internal energy (independent of the flow path |
|||
process) Q, W - infinitesimal quantities of heat and work, which, | |||
case, they depend on the path of the transition of the system from one state to another. | |||
Let the system do only mechanical expansion work. | |||
pdV. | |||
Substituting (1.6) into (1.5) we get | |||
When the isochoric process is V = const dV = 0 and integrating (1.7) we get | |||
in the isobaric process p = const, integration (1.7) and simple transformations give

From (1.8) and (1.10) it follows that in the isochoric and isobar processes the heat acquires the properties of the state function, i.e. does not depend on the path of the process. This provision is called gI's law Hess. The initial and final state of the chemical
stocks are starting materials and reaction products. The enthalpies of simple substances,
stable under standard conditions (298K and 0.10113 MPa) are assumed to be zero. For complex substances, the change in enthalpy is considered when they are formed from elements.
Ca (tv.) + C (tv.) + 1.5O2 = CaCO3 | |||||||||||||||
The rule for calculating the heat of reaction by the enthalpies of formation of substances follows from |
|||||||||||||||
hess's Law. | |||||||||||||||
stoichiometric coefficients of the reaction products and starting materials |
|||||||||||||||
respectively. However, in accordance with the agreement on signs, if | 0 then reaction |
||||||||||||||
exothermic (generates heat).
4. Heat capacity. The dependence of the thermal effect of the reaction on temperature
Heat capacity - is called the amount of heat required to heat a unit
the mass of the substance is 1 K. Distinguish the specific imolar thermal capacity, i.e. the amount of heat required to heat 1 kg. Or 1 mole of substance per 1 K.
The true molar heat capacity is determined as follows.
C (1.12) dT
where C is the molar heat capacity, mol K.
For heat capacities at constant volume and pressure (isochoric and isobaric) with
considering (1.8) and (1.10) we get
; C p | ||||||

Consider the dependence of the heat of the process on the temperature at a constant volume or pressure. Considering equations (1.13) we get
Equations (1.14) are called kirchhoff equations.
The change in heat capacity during the reaction is determined by the expression
i Cp, i (prod) | jC p, j (ref), | ||
those. the difference between the sum of the heat capacities of the reaction products and the starting materials.
From equation (1.14) it follows that if the change in heat capacity during the reaction is
it is reasonable (i.e., the heat capacity of the products is less than the heat capacity of the starting materials) and then the thermal effect of the reaction becomes more negative, the reaction becomes more efficient.
zothermic
To calculate the thermal effect of the process at T 2 (p = const), the Kirchhoff equation |
|||||
fa must be integrated. | |||||
Cp dT, | |||||
It must be borne in mind that in the temperature range T 1 - T 2 | there should be no phase transitions |
||||
dov substances. | |||||
Usually T 1 choose 298K, then | r H 0 - the thermal effect of the reaction in the stan- |
||||
dart conditions calculated by (1.11).
The heat capacity of real substances in a complex manner depends on the temperature, therefore
the following approximations are used to integrate the Kirchhoff equation: | ||||
Zero - the heat capacity of the products is equal to the heat capacity of the original substances, |
||||
those. C p 0 and the thermal effect of the reaction does not depend on temperature. | ||||
First order - C p | a const, then | |||
a T2 T1 | ||||
Molecular energy of burning
Most combustion processes are combustible, containing hydrogen and carbon, with oxygen from the air.
Before proceeding to the consideration of the physical and mathematical foundations of the theory of combustion, let us try to understand at the molecular level where the energy of combustion comes from, from the release of which everything else depends: the heating of the gas, the appearance of active chemical centers in it, etc.
Let us see what the heats of the main reactions of combining carbon and hydrogen with oxygen of the air are made of.
We write in accordance with the data table. 3.1 energy balance of the reactions of successive oxidation of solid carbon, for example graphite:
Thus, in the total solid carbon oxidation reaction, 386 kJ / mol are released:
Close in size and the energy released by combining hydrogen with oxygen:
The CO molecule is perhaps the most durable; its binding energy is 1016 kJ / mol. (The next in strength is the N2 molecule with a bond energy of 892 kJ / mol. There are three pairs of binding electrons in both molecules, in the chemical language there are three valence bonds. In the CO molecule, first one electron passes from O to C, then O + and C- become similar to nitrogen atoms; this is confirmed by the presence of a dipole moment in the CO molecule.) In the CO2 molecule, the bond of the second oxygen atom is weaker: according to Table. 3.1
The binding energies of oxygen in these compounds are comparable to the binding energy of the original oxygen molecule. Because
then only 240 kJ / mol per oxygen atom. In the low binding energy of the oxygen molecule, the reason for its chemical activity and the reason for using oxidation as an energy source.
The binding energy of a carbon atom in the crystal lattice of graphite (as well as diamond and amorphous carbon) is very high. The relatively small reaction energy C (s) + 0.5O2 = = CO + 98 kJ / mol is the difference of two very large quantities: one should subtract half of the O2 gap energy per atoms from the CO bond energy (256 kJ / mol) (59 kJ / mol) ) and subtract the heat of vaporization of the carbon atom. Actually, the heat of evaporation equal to 671 kJ / mol is also determined. It is also a very large quantity.
The conversion of solid carbon and gaseous hydrogen to hydrocarbon fuels occurs with a small change in energy. On the other hand, when oxygen is introduced into organic molecules of tina alcohols, aldehydes and ketones, organic acids, carbohydrates, almost as much energy is released as it is released during complete combustion (to CO2 and H2O), naturally, with the consumption of an equal amount of oxygen. Therefore, it can be approximated that with the complete combustion of any organic fuel, 419-500 kJ / mol of consumed oxygen is released. The only exceptions are some endothermic, energy-rich compounds, such as acetylene and dicyan, for example, their heat of combustion is greater.
Incomplete combustion is energetically disadvantageous not only in the calculation of the fuel molecule, but also on the spent oxygen molecule. In the reaction 2Q (s) + O2 = 2CO, only 210 kJ / mol is released instead of 466 when hydrogen is burned and 526 when CO is burned.
The strong bond of atom C in solid carbon leads to the fact that carbon does not evaporate. Carbon leaves the solid state only with oxygen in the form of CO or CO2.
In case of incomplete combustion and low temperature, the reaction 2СО = СO2 + С (tv)) + 41 kJ / mol is energetically advantageous only with respect to solid carbon. When calculating the free carbon atom, the corresponding reaction 2CO = СO2 + С - 129 kJ / mol has a large energy barrier. Therefore, soot and soot during combustion are formed only from the decomposition of organic molecules that have a carbon skeleton, but not from CO.
We now turn to the oxidation reactions involving nitrogen.
The nitrogen molecule N2 is very strong - its dissociation energy is 226 kJ / mol. Therefore, the reaction of converting N2 and O2 to 2NO is endothermic and, for thermodynamic reasons, can only take place at a high temperature.
The formation of higher oxides (NO2, N2O3, N2O4, N2O5) from nitrogen and oxygen proceeds with virtually no change in energy (as compared to the binding energy of N2 and O2). Therefore, from an energy point of view, oxygen packaged in compounds with nitrogen (CH3-ONO2 - nitro ester, CH3 (CeH2) (NO2) 3 - trinitrotoluene) is almost equivalent to gaseous oxygen. The oxygen integrated into the organic molecule, but bound to nitrogen, makes it possible to create substances that emit a lot of energy when the molecule is rearranged to form N2 and oxygen is transferred to the CO2 and H2O molecules. For this reason, compounds in which oxygen is bound to nitrogen (as well as to chlorine, in the groups СЮ3 and СlO4) are used as powders and explosives.
These are the general ideas about the molecular energy of combustion.

 Reception of indications of counters of hot and cold water supply
Reception of indications of counters of hot and cold water supply Calculation of the area of air ducts of various shapes and fittings
Calculation of the area of air ducts of various shapes and fittings What will happen if you do not transmit meter readings
What will happen if you do not transmit meter readings