High water hardness. Water hardness index
People in different countries for a long time came to the need to ration it, because high rigidity is bad: the pipes are clogged and it is impossible to wash them properly. But they began to do this in each country in their own way, who how, based on traditional units of measurement and methods for determining calcium and magnesium ions, because there were no standardized international units.
It is known that there is nothing worse than bad habits - it is very difficult to get rid of them! In coffee literature (although the rigidity is in essence a concept not from the field of coffee!), Different countries still measure stiffness in degrees, and in each country in their own, different from all others. Only Russian and German degrees of rigidity are identical, true, long ago abolished in both these countries, but stubbornly existing in the definition of concepts.
In the USSR, until 1952, the degrees of hardness used coincided with the German ones. In Russia, the normal concentration of calcium and magnesium ions, expressed in milligrams of equivalent per liter (mEq / l), is used to measure stiffness. One mEq / l corresponds to a content of 20.04 milligrams of Ca2 + per liter of water or 12.16 milligrams of Mg2 + (atomic mass divided by valence).
In other countries, it is customary to denote stiffness in CONDITIONAL degrees:
German degrees (dGH)
1 ° = 1 part of calcium oxide — CaO in 100,000 parts of water, or 0.719 parts of magnesium oxide — MgO in 100,000 parts of water, or 10 mg of CaO in 1 liter of water, or 7.194 mg of MgO in 1 liter of water. dGH (dH) and dKH are currently most commonly used in aquarism as a unit of measurement for stiffness, the designation dGH refers to total hardness, dKH to carbonate;
French degrees (fh)
1 ° = 1 part CaCO 3 in 100,000 parts water, or 10 mg CaCO 3 in 1 liter of water;
American degrees (usH)
1 ° = 1 grain (0.0648 g) CaCO 3 in 1 gallon (American! 3.785 l) of water. Dividing grams per liters we get: 17.12 mg / l of caso 3. However, there is another definition of the American degree: 1 part of CaCO 3 per 1,000,000 parts of water (in the English-language literature, the expression of concentration as 1 part per 1,000,000 parts is called ppm - part per million (one part per million), and is often used. In practice it is identical to 1 mg / l). So this 1 American degree = 1 mg CaCO 3 in 1 liter of water. It is this value of the American degree adopted in all tables with transition coefficients for converting some units of measurement of rigidity to others.
English degrees (Clark)
1 ° = 1 gran (0.0648 g) in 1 gallon (English! 4.546 l) of water = 14.254 mg / l CaCO 3.
Feel like everything is not easy ?! Therefore, I will give a table that allows you to compare and translate some degrees of rigidity to others:
Table 1
| Name of units | Mg eq / l | Degree of rigidity | |||
|---|---|---|---|---|---|
| Deutsch | French | American | English | ||
| 1 mEq / l | 1 | 2.804 | 5.005 | 50.045 | 3.511 |
| 1 German degree dH | 0.3566 | 1 | 1.785 | 17.847 | 1.253 |
| 1 french degree | 0.1998 | 0.560 | 1 | 10,000 | 0.702 |
| 1 American degree | 0.0200 | 0.056 | 0.100 | 1 | 0.070 |
| 1 English degree | 0.2848 | 0.799 | 1.426 | 14.253 | 1 |
Water hardness is a traditional measure of the ability of water to react with soap: hard water requires a significant amount of soap to form a foam. Deposition of scale in hot water pipes, boilers and other household appliances is caused by hard water. Water hardness is caused by dissolved ions of polyvalent metals. In fresh water, the main ions that cause hardness are calcium and magnesium; ions of strontium, iron, barium and manganese are also important. Water hardness is usually determined by the reaction of polyvalent metal ions present in water with chelating agents, for example, EDTA, and is expressed as the equivalent concentration of calcium carbonate. Stiffness can also be assessed by determining individual concentrations of the components forming it, the sum of which is expressed in terms of an equivalent amount of calcium carbonate. The degree of hardness of drinking water is classified on the basis of equivalent concentrations of CaCO 3 in it, as follows:
Soft - 0-60 mg / l
Average hardness - 60-120 mg / l
Hard - 120-180 mg / l
Very hard - 180 mg / l and above.
Stiffness is also classified based on equivalent concentrations of CaO or Ca (OH) 2. In the SI system it is recommended to express the rigidity in moles of Ca 2+ per m 3.
Despite the fact that hardness is determined by cations, it can also be considered as carbonate (disposable) and non-carbonate (constant) hardness. Carbonate hardness indicates the amount of carbonates and bicarbonates in solution, which can be removed or precipitated by boiling. This type of stiffness is responsible for the deposition of scale in the hot water pipes and boilers. Non-carbonate hardness is caused by a combination of hardness ions with sulphates, chlorides and nitrates and is defined as “permanent hardness”, since it cannot be eliminated by boiling.
Alkalinity, as an indicator of water buffering, is closely related to hardness. Alkalinity is mostly caused by anions or molecular forms of weak acids, mainly hydroxides, bicarbonates and carbonates; in the presence of other forms of water, such as borates, phosphates, silicates and organic acids, they also make a small contribution to the indicator of alkalinity of water. Regardless of which dissolved forms ensure the alkalinity of water, it is always expressed as an equivalent amount of calcium carbonate.
In cases where the alkalinity of surface waters is due to the presence of carbonates and / or bicarbonates, its value is usually close to the value of hardness.
Spread of hard water
The main natural sources of water hardness are sedimentary rocks, filtration and runoff from the soil. Hard water is usually formed in areas with a dense topsoil and calcareous formations. Groundwater is usually characterized by greater rigidity than surface water. Underground waters rich in carboxylic acids usually have a high solubility with respect to soils and rocks containing measurable amounts of the minerals calcite, gypsum and dolomite, as a result of which hardness levels can reach several thousand mg / l.
The main industrial sources of stiffness are effluent from enterprises producing inorganic chemicals and the mining industry. Calcium oxide is used in the construction industry in lime mortar, plaster and other materials. It is also used in the production of paper pulp and paper, sugar refining, oil refining, tanning, water and wastewater treatment. Magnesium is also used in various processes in the textile, tanning and paper industries. Magnesium alloys are widely used in foundry and stamping production, portable machines, luggage equipment and household products of wide application. Magnesium salts are also used in the production of metallic magnesium, fertilizers, ceramics, explosives and medicines.
Impact on hard water on health
As noted in the article on calcium and magnesium, the main factors determining the hardness of water are calcium and magnesium ions. Data on adverse health effects specifically associated with high levels of calcium or magnesium in drinking water are not available.
In addition to domestic discomfort resulting from the use of water with a high degree of hardness, another possible inconvenience may occur when magnesium is bound to the sulphate ion, as a result of which water acquires laxative properties.
The calcium ion taste threshold in drinking water varies from the anions present; for magnesium ion, the taste threshold is less. Further details regarding the relationship between water hardness and cardiovascular diseases can be found in Part III, where health aspects of the inorganic components of water are considered. Recommended water content values for calcium and magnesium are not suggested, as this value is suggested for general hardness based on aesthetic considerations.
Other aspects
Soft water is more prone to cause pipe corrosion, and as a result, some heavy metals, such as copper, zinc, lead and cadmium, may be present in drinking water in the distribution system. The degree of corrosion and dissolution of metals is also a function of pH, alkalinity, and dissolved oxygen concentration. In some localities, corrosion is so strong that special precautions have to be taken in the water supply system.
In areas with very hard water, house pipes can become clogged with deposited scale; hard water also forms scum on cooking utensils and increases soap consumption. Thus, hard water can be not only unpleasant, but also economically burdensome for the consumer. The perception of water hardness by the population is not the same in different localities, it is often associated with the hardness to which the consumer has been used for a number of years, and in many localities water with hardness does not cause objections of more than 500 mg / l. Although, an acceptable balance between corrosion and scale problems provides a hardness level of approximately 100 mg CaCO 3 / L.
As you know from a school chemistry course, ordinary water contains calcium and magnesium ions. The high content of Ca 2+ and Mg 2+ ions gives the water a negative quality, called stiffness
CaCO 3 + CO 2 + H 2 O = Ca (HCO 3) 2
MgCO 3 + CO 2 + H 2 O = Mg (HCO 3) 2
This process is widely carried out in natural conditions, leading to the removal of eroded limestone to surface waters, and then to the seas and oceans.
Non-carbonate (constant) stiffness due to the presence of sulfates, chlorides of magnesium and calcium in water, as well as other salts (MgSO 4, MgCl 2, CaCl 2).
Total hardness = carbonate (temporary) hardness + non-carbonate (constant) hardness.
In everyday life, anyone can face the task of measuring the hardness of water at home. Stiffness adversely affects the quality of various processes that use water with increased stiffness. The lower the percentage of dissolved salts in water, the softer and healthier the water. Dishwasher operation, the amount of washing powder, the quality of water in the aquarium, the need to install a water softener, etc. In general, there are many goals.
In Russia, hardness is measured in “degrees of hardness” (1 ° W = 1 mEq / l = 1/2 mol / m3). Abroad adopted other units of measurement of water hardness.
Stiffness Units
1 ° W = 20.04 mg Ca 2+ or 12.15 Mg 2+ in 1 dm 3 of water;
1 ° DH = 10 mg CaO in 1 dm 3 of water;
1 ° Clark = 10 mg CaCO 3 0.7 dm 3 of water;
1 ° F = 10 mg of CaCO 3 in 1 dm 3 of water;
1 ppm = 1 mg CaCO 3 per 1 dm 3 of water.
According to the intensity of the formation of scale in the kettle, certain conclusions can be drawn: the more plaque, the harder the water.
Comparative qualitative the conclusion about the hardness of water can be carried out as follows. On a glass slide, apply a drop of rain, boiled and boiled tap water. After drying on the intensity of the precipitation, you can make a conclusion about the hardness of your water. Rainwater is the softest, because there are practically no calcium and magnesium salts. The precipitate after evaporation of unboiled water allows to make a conclusion about the total hardness, and boiled one - about temporary hardness.
But at home you can accurately and quantitatively assess the hardness of the water. From the course of organic chemistry you know that laundry soap, like any other, is difficult to soap in hard water. This method is based on the fact that as soon as soap binds an excess of calcium and magnesium salts, soap suds appear. To determine the hardness of the water, you need to weigh one gram of soap, grind it and gently, so that no foam is formed, dissolve in a small amount of hot distilled water. Distilled water can be purchased at pharmacies or in auto shops. It is used to add to the battery when the electrolyte concentration increases.
Next, pour the soap solution into a cylindrical glass and add distilled water to the level of 6 centimeters, if the soap is 60% or to the level of 7 centimeters, if the soap is 72%. The percentage of soap is indicated on the bar. Now every centimeter of the level of the soap solution contains the amount of soap capable of binding hardness salts, the amount of which corresponds to 1 ° dH in 1 liter of water. Next, in a liter jar pour half a liter of the investigated water. And continuously stirring, gradually add the prepared soap solution from the glass into the jar with the test water. At first, only gray flakes will be on the surface. Then multi-colored soap bubbles will appear. The appearance of a stable white soap suds suggests that all hardness salts in the water under study are connected. Now we look at our glass and determine how many centimeters of the solution we had to pour out of the glass into the test water. Each centimeter bound in half a liter of water a number of salts, corresponding to 2 ° dH. Thus, if you had to pour 4 centimeters of soap solution into the water before the foam appeared, then the water hardness is equal to 8 ° dH.
If you poured all the soap solution into the water, and the foam never appeared, this means that the hardness of the water under study is greater than 12 ° dH. In this case, the test water is diluted with distilled water twice. And we are analyzing again. Now the result of stiffness will need to be multiplied by two. The resulting value will correspond to the hardness of the investigated water.
According to the table you can determine the quality of the investigated water:

It is not possible to determine the rigidity with an accuracy of up to a thousandth of a degree, but it is quite possible to estimate the sharp departure of the overall rigidity from the norm with an accuracy of 1-2 ° dH Scatter readings of 1-2 degrees is quite acceptable. Given the simplicity and accessibility of the method, it can certainly be successfully applied.
Using this method, it is possible in the field to estimate the hardness of water from various water sources and to perform an interesting design and research work.
Sources:
1Rudzitis G.E. Chemistry. Inorganic chemistry. Organic chemistry. Grade 9: studies. for general education. organizations with on the electron. carrier (DVD): basic level / G.E. Rudzitis, F.G. Feldman. - M .: Enlightenment, 2013. - 224 pp., Ill.


 Flammable substances and materials it
Flammable substances and materials it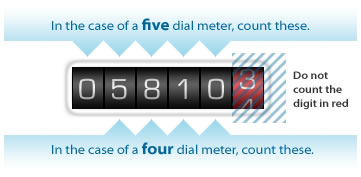 Transfer the gas meter readings to Nizhegorodenergogazraschen on gas nn
Transfer the gas meter readings to Nizhegorodenergogazraschen on gas nn The main chemical reaction of the combustion process
The main chemical reaction of the combustion process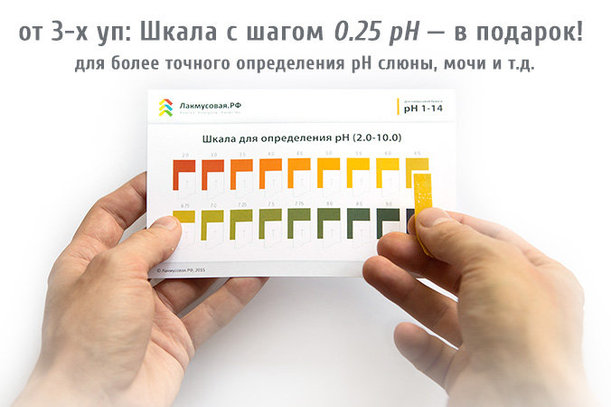 Litmus test - pH measurement
Litmus test - pH measurement