Burning is called. Chemical processes during combustion
Some basic definitions
The number of atoms and molecules is conveniently expressed in terms of quantities of substance or the number of moles of substance. One mole of matter corresponds to 6.023 ∙ 10 23 particles (atoms and molecules). The number N A \u003d 6.023 ∙ 10 23 mol -1 is called the Avogadro number. Mole fraction x icomponent i is the ratio of the number of moles n i component i to the total number of moles n \u003d ∑ n i mixtures ( x i \u003d n i / n).
Weight m is a fundamental property of a substance (the unit of measurement in the SI system is kilogram). The mass fraction Wi is the ratio of the mass m i component i to the total mass of the mixture m \u003d ∑ m i (Wi \u003d m i / m).
Molecular weight (or molecular weight) M i(g / mol) component I - is the mass of one mole of this component. So, for atomic carbon, molecular hydrogen, oxygen and methane we have: M c \u003d 12 g / mol, Mn 2 \u003d 2 g / mol, Mo 2 \u003d 32 g / mol, Mn 4 \u003d 16 g / mol. The average molar mass of the mixture M (g / mol) can be expressed in terms of the molar fractions of the components (M \u003d x i M i).
Determination of combustion processes
Combustion - it is difficult physical and chemical processin which combustible substances and materials under the influence of high temperatures enter into chemical interaction with an oxidizing agent (atmospheric oxygen), turning into products of combustion, and which is accompanied by intense heat and light emission.
The conditions necessary for the occurrence of the combustion process:
The presence of a combustible substance (GV);
The presence of an oxidizing agent (O.) - air oxygen;
The presence of a source of ignition (I.Z.).
The combustible material must be heated to a certain temperature at which the oxidation process will begin;
To heat a combustible substance to a certain temperature, a certain ignition source power is required;
To maintain the combustion process, certain concentrations of fuel and oxidizer are necessary.
The most important combustion processes are heat and mass transfer. The most common property of burning is the occurrence of a flame and moving it throughout the combustible mixture by transferring heat or diffusing the active particles from the combustion zone to a fresh combustible mixture.
Flame - This is a visible manifestation of burning. It is also called the burning zone. This is the part of the space where the transformation of the combustible mixture into products of complete and incomplete combustion occurs.
The main parameters of the combustion process, leading to death and causing material damage, are:
Large amount of heat;
Heat;
Toxic composition of combustion products.
The combustion process from the standpoint of the molecular kinetic theory of gases
Fig. 2.1. The proportion of active molecules depending on temperature: T 2\u003e T 1
When heated gas combustible mixture in it increases the excess energy.
The difference between the average energy level of molecules in the active state and the average level of the inactive state is called the activation energy. This can be represented graphically (Fig. 2).

Fig. 2.2. Energy diagram of the reaction course G.V. + O. ® PG: E act - activation energy; Q xr. - thermal effect of the combustion reaction
The energy released as a result of the interaction of the "first" reacted molecules is transferred to neighboring molecules. They are excited, the process is repeated around the reacted molecules with great frequency and intensity. A self-sustaining, self-accelerating to the entire reaction mixture (2H 2 + O 2) process of chemical interaction begins, accompanied by the formation of water molecules and the release of heat into the environment and accompanied by luminescence, i.e. arises and spreads the burning process.
The higher the numerical value of the E act, the more difficult it is to make this pair of components enter into chemical interaction. Therefore, the value of E act is an indirect indicator of the degree of fire danger of this chemical system.
Types and modes of combustion
Combustion can be classified by the following parameters:
1. According to the condition of combustible components mixing:
a) kinetic - combustion of pre-mixed gas or vapor-air mixtures. Since the mixture of fuel and oxidant is ready for burning until it ignites, the total rate of the combustion process depends only on the speed chemical reaction burning. If such a combustion occurs in a closed or limited volume, then an explosion may occur. Since the energy released during the combustion of the mixture does not have time to be discharged beyond this volume, by increasing the pressure, the structures may be destroyed;
b) diffusion, diffusion combustion is called combustion, when the formation of a combustible medium (a mixture of fuel and oxidizer) occurs before the combustion zone or in the combustion zone.
2. According to the intensity of the receipt of combustible components in the chemical reaction zone:
a) laminar, while the components of the combustible mixture enter the combustion zone relatively calmly. In this case, the numerical value of the Reynolds criterion, which characterizes the thermodynamic regime, will be significantly less than the critical one (Re<2300).
b) turbulent, with the components of the combustible mixture entering the combustion zone at high speed. The Reynolds number in this case is more than 2300.
3. According to the state of aggregation of the components of the combustible mixture:
a) homogeneous fuel and oxidizer are in the same aggregative state (gaseous);
b) heterogeneous (multiphase) - fuel and oxidant are in different aggregative states.
4. On the speed of propagation of the zone of chemical reaction of combustion:
a) deflagration (slow) distribution of the chemical reaction zone (speed from 0.5 to 50 m / s);
b) detonation (explosive), when the zone of chemical reaction of combustion propagates with the speed of a shock wave (from several hundred meters per second to several kilometers per second).
The space in which vapors or gases burn is called by flame .
Laminar flames pre-mixed. In laminar flames of a pre-mixed mixture, the fuel and oxidant are mixed before the start of combustion and the flow is laminar.
The flame of the pre-mixed mixture is called stoichiometric if the fuel (hydrocarbon) and oxidizer (oxygen - O 2) consume each other completely, forming carbon dioxide (CO 2) and water (H 2 O). If there is an excess of fuel, they say that the mixture is rich, and if there is an excess of oxidant, they say that the mixture is poor.
Consider the simplest examples:
1) 2H 2 + O 2 → 2H 2 O - stoichiometric mixture,
2) ЗН 2 + О 2 → 2H 2 O + Н 2 - rich mixture (Н 2 in excess),
3) CH 4 + ZO 2 → 2H 2 O + CO 2 + O 2 - lean mixture (O 2 in excess).
Each symbol in such a chemical reaction equation corresponds to one mole of a substance. Thus, the first of these equations means that two moles of H 2 react with one mole of O 2 to form two moles of H 2 O.
If the chemical reaction equation is written in such a way that it describes the reaction of just one mole of fuel, then the mole fraction of fuel in a stoichiometric mixture can be easily determined from the relation
x mountains, stokh \u003d 1 / ((1 + v)
Here vdenotes the number of moles of O 2 in the reaction equation with the formation of CO 2 and H 2 O. An example is the reaction
H 2 + 0.5O 2 → H 2 O, v \u003d 0.5, x H 2, stoch \u003d 2/3
If the oxidizer is air, then it should be taken into account that dry air contains only 21% oxygen, as well as 78% nitrogen and 1% noble gases. Thus, for air, X N 2 \u003d 3.762 X O 2. From here molar fractions for stoichiometric mixture with air will be equal
x mountains, stokh \u003d 1 / (((1 + v ∙ 4.762), ![]() ,
,
where v as before means the number of moles of O 2 in the reaction equation of complete conversion of one mole of fuel to CO 2 and H 2 O. Some examples of values v and molar fractions of fuel for stoichiometric mixtures of fuel with air are shown in Table 1.
Pre-mixed mixtures of fuel and air (in this case, an appropriate amount of N 2 must be added to the reaction equation, see Table 1) are characterized by the equivalent ratio for air:
λ \u003d (x w / x mountains) / (x w, stoch / x mountains, stoh) \u003d (w w / w mountains) / (w w, stoch / w mountains, stoch)
or the reciprocal of - the equivalent ratio for fuel F (F \u003d 1 / λ). This formula can be transformed in order to be able to determine the magnitude of the molar fractions of the mixture by value F:
x mountains \u003d 1 / ((1+ (4,762 ∙ v) / F), x w \u003d 1 - x mountains,
X weight / 4,762, \u003d ∙ 3,762
Examples of v values \u200b\u200band mole fractions of fuel x mountains, stoichi for stoichiometric mixtures of fuel with air
irrigation is always accompanied by chemical transformations. Combustion in air - the interaction of a combustible substance with oxygen. However, it should be borne in mind that oxides of nitrogen, halides, and ozone can act as oxidizers in the combustion process. Known combustion processes occurring with the participation of only one source of the product - compounds capable of rapid decomposition. Examples of such compounds are acetylene and hydrazine.
Chemical processes occurring during combustion are extremely complex. Even for the simplest case - the combustion of hydrogen in oxygen, the generalized equation of which has the form
2H 2 + 0 2 \u003d 2H 2 0,
several dozen elementary stages have been established and studied.
To date, the mechanisms of chemical transformations during combustion of only a few substances, such as hydrogen, carbon monoxide, methane and ethane, have been studied in sufficient detail. This knowledge is used to predict the conditions of ignition and combustion of many substances.
2.1. Chain reactions
Chain reactions, in contrast to ordinary chemical transformations, are characterized by the appearance in each elementary act of an active particle: an atom with an unpaired electron, a free radical or an ion. The appearance of active particles causes a chain of transformations of the starting materials into reaction products. Atoms, free radicals and ions are more reactive than valence-saturated molecules. Therefore, despite the considerable energy costs required to break chemical bonds and the formation of active particles, the chain development of a reaction often turns out to be energetically more beneficial than the direct interaction between molecules.
Combustion processes mainly occur through a chain mechanism. Chain reactions - complex reactions that take place in several stages, representing:
The origin of the chains (initiation), in which the active particles are formed;
The continuation of the chains, in which the active particles enter into chemical interaction with the starting materials, as a result of which new active particles are formed;
Breakage of chains in which the "death" of the active particles occurs with the formation of the final reaction products
The origin of the chains can occur under various conditions. For example, as a result of dissociation of molecules under the action of thermal energy, or ionizing radiation, in an electric discharge. The death of active particles occurs when they recombine *, when free radicals interact (homogeneous chain breakage), when active particles interact with solid surfaces (heterogeneous chain breakage) or as a result of their reaction with combustion inhibitors.
There are unbranched and branched chain reactions. In the unbranched for each active particle consumed in the reactions of continuation of the chain, there is one re-emerging. The number of product molecules formed per initial active particle is called the chain length. The length of chains in combustion reactions ranges from hundreds of thousands to tens of millions. The rate of unbranched chain reactions can be affected by minor admixtures of substances that can effectively interact with active particles, such as combustion inhibitors.
Some substances - initiators - facilitate the formation of active particles and thereby accelerate unbranched chain reactions.
In branched chain reactions to one active particle, consumed with the continuation of the chain, two or more active particles are formed. One of them continues the primary chain, while others begin new chains, forming a branching (fig. 2.1).
"Recombination is the process of the formation of neutral atoms or molecules from charged particles. The atoms and molecules formed during recombination can be in the ground or excited state.
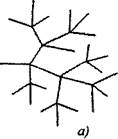
Fig. 2.1. Schematic representation of the reaction chains:
a) branching in each link of the chain
6) rare branching
For example, during the combustion of hydrogen in the chain initiation reaction:
an active atom is formed. In the chain continuation reaction:

there is an increase in the number of active atoms that are the beginning of new chains.
Branched chain reactions can proceed in a stationary mode, in which the speed of branching is less than the rate of death of the active particles, and in non-stationary, in which death occurs more slowly than branching. In the latter case, the speed of the chain reaction increases exponentially and is limited only by the consumption of the starting materials. The transition from stationary to non-stationary mode occurs abruptly with a slight change in the conditions of the reaction: temperature, pressure or concentration of one of the reactants. Such a rapid acceleration is considered as self-ignition of the reaction mixture or a chain explosion.
The discovery of branched chain reactions was of great importance for the creation of a theory of combustion processes. It is proved that there are two types of ignition: thermal and chain. Patterns set in
Chapter 2. Chemical processes at burning
the theory of chain processes, can effectively influence the development and suppression of combustion processes during fires.
The origin of the chains.The process of nucleation of the initial active centers plays a large role in the development of unbranched chain reactions. It compensates for the loss of active centers during chain termination reactions. This mechanism determines the conditions for the formation of a stationary regime in the initial period of accumulation of active centers. With a low rate of initiation, this period can be significant.
Most of the chemical reactions in flames have a significant energy reserve of active centers. Under these conditions, the initiation of active centers is associated with overcoming a significant energy barrier.
In this case, an important role is played by factors that ensure a significant rate of emergence of active centers: chemically active additives, radiation, electrical discharge, radioactive decay products.
Among the factors that greatly facilitate the generation of active centers, heterogeneous reactions should be noted. In the molecules of combustible substances adsorbed on a solid surface, interatomic bonds are weakened and their breaking requires less energy expenditure. Under these conditions, the rate of generation of active centers is significantly higher than in the gas volume. The activation energy in the heterogeneous interaction of the components of the combustible mixture is also lower than in the case of a homogeneous one. Heterogeneous initiation of active centers under conditions of actual combustion processes is an important factor in the accelerated achievement of a stationary regime with unbranched chain processes.
Some features characterize unbranched chain reactions that occur with the participation of atomic components. In the absence of impurities interacting with active centers without regeneration, chain termination becomes possible only when atoms recombine by triple collisions and on the surface.
At any temperature, all gaseous substances are partially dissociated. Some of the molecules break down into atoms. In this case, there is an equilibrium between the processes of dissociation and the union of atoms into molecules. The degree of dissociation exponentially depends on temperature.
If there are no impurities in the combustible mixture breaking the chains, the concentration of the atomic components of the reaction remains practically unchanged. The atoms that entered into the reaction are immediately regenerated in the same
Korolchenko A.Ya. Combustion and explosion processes
lichie. The initial initiation compensates for the reacted atoms in the same amount as in the non-reactive system. Stationary reaction does not affect this process. The balance of one of the components of the reaction, which is distinguished by the least strong bond between the atoms in the molecule, is a characteristic feature of this mode. The concentration of another atomic component in this case is quasistationary, but more equilibrium.
With the homogeneous initiation of combustion reactions, the stationary regime will be established only after a certain period of time, since dissociation requires a large activation energy. During this period, the dissociation rate exceeds the recombination rate, and the active centers accumulate in the reaction system. The rates of both processes are compared only after increasing the concentration of active centers to equilibrium. This time period is called the induction period.
The presence of a solid surface as a catalyst in the reaction zone does not change the state of thermodynamic equilibrium. The catalyst equally affects the forward and reverse reactions. Active centers not only originate on a solid surface, but also break off on it. However, the presence of a catalyst accelerates the achievement of an equilibrium dissociation state.
If active substances are present in the combustible mixture, which are able to participate in chain breakage reactions, they reduce the concentration of active centers. In this case, the equilibrium dissociation of one of the initial components is disturbed, which slows down the reaction and may lead to its termination.
Experiments show that when initiating an unbranched chain reaction by an external source (for example, a light source), the concentration of active centers at the initial stage of the combustion process development can significantly exceed the equilibrium one.
During the course of branched chain reactions, the conditions of initial initiation have a significant impact on the development of the reaction. In slow-moving processes, the addition of a partially reacted mixture to the initial one shortens the induction period and accelerates the moment of flame combustion.
The continuation of the chains.A characteristic feature of unbranched chain reactions is the quasi-stationarity of the concentration of active centers. In the absence of an open circuit, the active particles are formed in
Chapter 2. Chemical processes during combustion
the same amount in which they are spent. New arise only at the initial initiation. With equal rates of generation of active centers and chain termination, a constant concentration of active centers and a stationary reaction mode are established. The reaction rate will decrease as the initial components are consumed.
In the case of a branched chain reaction, the concentration of active centers in the reacting system increases regardless of the conditions of their initial initiation. Implemented self-accelerating reaction mode, which has an avalanche character. In this case, for the complete transformation of the initial components into the final products of the reaction, one initial active center is sufficient.
The kinetic equation of a branched valuable reaction is as follows. The change in the concentrations of the stable starting components over time can be neglected to a first approximation, and only faster changes in the concentration of active centers can be taken into account. p.Changes in this speed are determined by the rate of initial initiation and the ratio of the rates of the reactions of branching and chain termination. The initial initiation rate does not depend on the concentration of active centers present in the system. The rates of branching and breakage processes are proportional to the concentrations of active centers. Under these conditions, the overall balance of the formation and expenditure of active centers is determined by the sum of the rates of the processes of initiation, branching, and breakage:
![]() (2.1)
(2.1)
where and are the rate constants of the branching and breakage reactions. Denoting, we get:
![]() (2.2)
(2.2)
When the time derivative of the concentration of active centers
is positive. The reaction rate increases with time. This feature of branched chain reactions is due to the multiplication of active centers in such regimes when the rate of the branching reaction exceeds the rate of the chain termination reaction.
Korolchenko A.Ya. Combustion and explosion processes
If before the start of the reaction the system did not contain active centers, i.e. att\u003d 0, n \u003d 0 integration of equation (2.2) gives:
![]() (2.3)
(2.3)
The total reaction rate of CO is determined by the rate of the branching process. The final products are formed only during this reaction. For each elementary chain branching event, molecules of the final product are formed. Therefore:
![]() (2.4)
(2.4)
The development of a chain reaction in time is determined by the ratio of the rate constants of the reactions of branching and chain termination, and when the exponent in equation (2.4) is positive and re-
the action is unlimitedly accelerated. In the initial period of development of the reaction, the following relationship holds true:
Due to the fact that the rate of initial initiation is small, there is no noticeable chemical transformation in the initial period. After some time, the value becomes substantially greater than one. After that, the reaction rate in accordance with equation (2.4) begins to increase rapidly and reaches very large values, although it was practically imperceptible before.
The presence of a delay period (induction period) during the development of a chain reaction is due to the necessity of accumulating a certain number of active centers in the reacting system. Only after this chemical transformation becomes noticeable.
The magnitude of the induction period in chain reactions determines the ratios of the rates of branching and chain termination processes, not the rate of initial initiation. In turn, the rates of branching and breakage reactions are due to the chemical features of each reacting system; they are determined by dependencies on temperature and concentrations of the starting components. The peculiarity of chain reactions for
is that the branching processes require significant activation energy, while the temperature coefficient of the rate constant of the breakage process is close to zero. In the reactions of termination of chains of all three types: in the case of bulk and heterogeneous recombinations, in the interaction of radicals with active impurities, the activation energies are zero.
With an increase in temperature, the total pressure of the mixture, or a change in the concentration of the reacting components, a change in the rate constants of branching and breakage is possible, at which the reaction changes from stationary to non-stationary. The peculiarity of this process lies in the abrupt transition from one mode to another, in changing the reaction rate from a negligible value to an unlimitedly increasing one.
The flow of some chain reactions is accompanied by the formation of intermediate products that are comparatively stable, but with the ability to generate active centers. Such reactions include, for example, hydrocarbon combustion reactions, as intermediates in which peroxides and aldehydes are formed. This leads to branching of the chain. However, due to the relative stability of intermediate products, the acceleration of the reaction stretches over time. Such slow branching chains are called degenerate.
Chain reactions with the usual radical branching mechanism, as a rule, due to the high activity of the radicals, proceed quite quickly. The resulting radicals either initiate a fast-accelerating reaction, or recombine and exit the process.
Open circuit The active particle, like any gas molecule, makes random motions inside the reacting system, colliding with other molecules. At the same time, there is a certain probability at some collision of interaction with another active particle or molecule and the formation of a new active particle, continuing the path of the previous one. The development of the reaction chain is similar to the Brownian motion of inert molecules, although the transfer of active centers is accompanied by a chemical reaction. On the path of development of the chain alternate active centers of two or more types.
Korolchenko A.Ya. Combustion and explosion processes
The chain of reactions continues until the moment when the active particle does not react without regeneration. In this case, the so-called open circuit occurs. Breakage processes play a large role in the kinetics of chain reactions. There are two types of reactions leading to the death of active centers:
Homogeneous break (death in the volume of the reaction mixture);
Heterogeneous break (death on a solid surface) Homogeneous break of chains is possible with one of two processes:
when radicals recombine or when various chemically active components interact with active centers without regeneration of the latter.
Heterogeneous chain breakage occurs on soot particles formed during combustion, or on the surface of solid burning materials. An open circuit on a solid surface can be considered as the diffusion of active centers from the gas mixture to this surface on which they disappear. The mechanism of recombination on a solid surface is that the active particle, which has an increased reactivity, is sorbed * on the surface. The radicals that are sorbed on the adjacent sites recombine with each other, since there are no energy and spatial obstacles for this process. The molecules of stable compounds formed as a result of recombination are no longer involved in the development of a chain reaction.
However, not every collision of an active particle with a solid surface leads to its adsorption. Perhaps its reflection from the surface. The probability of adsorption of an active center by a solid surface is called the accommodation coefficient. This coefficient is a characteristic of the chemical affinity of the active particle and the surface. In practically important cases, the active particle after reflection from the wall does not move away from it far. There is the likelihood of new collisions with the wall until its accommodation occurs. Because of this process, under certain conditions, the reaction rate is practically independent of the accommodation coefficient. The process proceeds in such a way as the EU
* Sorption - absorption by a solid body (or liquid) of a substance ha of the environment. The absorbing body is called a sorbent, absorbed- sorbate. Distinguish the absorption of the entire mass of the sorbent (absorption) and the surface layer (adsorption). Sorption due to the interaction of the chemical type between the surface of the solid sorbent and the sorbate is called chemisorption.
__________________________ Chapter 2. Chemical processes during combustion
if an open circuit occurred at every collision. The concentration of active centers at the surface can be taken to be zero.
In the absence of active impurities in the reacting mixture, the breakage of the chains can occur either on solid surfaces or homogeneously by recombination of radicals in the bulk. In real fires, the second way is mainly implemented.
Certain influence on the kinetics of chain reactions have additives in the reacting system of inert gases. Inert additives increase the number of collisions with active particles, increase the likelihood of chain breakage and, accordingly, inhibit the overall reaction.
More effective inhibition of chain reactions is achieved with the introduction of reactive additives - inhibitors into the reacting mixture. Inhibitors interact with the active centers, leading the reaction, breaking the chain. The kinetics of the reaction in the inhibited mixture is determined by the conditions of competition between the inhibitor and the main components of the reaction when interacting with active centers. With the high efficiency of the inhibitor and the moderate rate of generation of new active centers, already small additives of the inhibitor can completely suppress the course of the chain reaction.
The processes of inhibition are of great importance in the practice of fire and explosion safety. The use of inhibitors allows you to effectively influence the combustion processes.
2.2. Chemical processes during the combustion of hydrogen
The interaction of a hydrogen molecule with an oxygen molecule proceeds in a complex way through a series of successive stages. It is now firmly established that the combustion of hydrogen occurs by a chain mechanism, with particles playing the role of active centers. The sequence and significance of elementary reactions during the combustion of hydrogen are described in great detail for various conditions for the emergence and development of hydrogen flames.
The most detailed analysis of the hydrogen combustion process in the autoignition mode using experimental and computational methods was performed by Professor A. N. Baratov. He proposed the following scheme of the process, including fourteen basic elementary reactions:
Korolchenko A.Ya. Combustion and explosion processes
The origin of the active centers occurs by reaction
![]()
Continuation of the chain of reactions
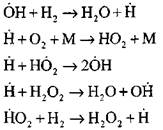
Branching chains
 |
open circuit
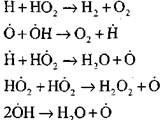 |
The concentration of hydrogen atoms in the initial stage of the autoignition process is an insignificant part of the initial hydrogen content. With the development of a chain reaction, the rate of conversion of molecular hydrogen becomes so high that it is consumed in hundredths of a second.
2.3. Chemical reactions when burning carbon monoxide
The interaction of carbon monoxide with oxygen is a major reaction for combustion processes. The course of this reaction during
__________________________ Chapter 2. Chemical processs at burning
many cases determines the laws of combustion of carbon-containing substances. The reaction is characterized by a branched chain mechanism. It has a number of features.
For a long time there was a belief that a completely dry mixture of CO and 0 2 could not ignite and burn. However, carefully set experiments in which the absence of water was monitored using a mass spectrograph showed that ignition is also possible for a dry mixture. It should be noted that the presence of CO + 0 2 water vapor or hydrogen in the system activates the process of ignition and combustion by increasing the number of possible active centers. The accelerating effect of water is especially noticeable at low concentrations.
Combustion of carbon monoxide in the presence of water vapor or small additions of hydrogen occurs with the participation of the following elementary processes:

The radicals, H0 2, which are formed by reaction (VI), can continue the chain (reaction VIII) or lead to its breakage by reaction (IX-XII).
To assess the conditions for the transition of slow oxidation of CO into a chain explosion, let us estimate the probability of a chain break through the radical H0 2; here we take into account that the role of reactions (X) and (XI) in the chain termination will be insignificant in terms of
compared with reaction (IX), since the rate constants of the processes (IX-XI) at temperatures of the order of 1000 K are close to each other, but the concentration of radicals is much lower than the concentration of hydrogen atoms, since radicals have a higher chemical activity. Therefore, the probability of an open circuit through the radical H0 2 can be written in the form:
At a temperature of 1000K
Therefore, provided that
![]()
![]()
magnitude effect
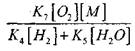
on solving equation (2.7) will be weak.
Chapter 2. Chemical processes during combustion
When ![]() or
or ![]() (what is observed in
(what is observed in
real flames WITH), equation (2.7) is converted to the form:
![]() (2.8)
(2.8)
Thus, the condition of burning carbon monoxide in the air is largely determined by the presence and patterns of hydrogen burning. The oxidation of CO by reaction (I) depends on the concentration of OH radicals formed in the reactions involving hydrogen.
The peculiarity of the combustion reaction of carbon monoxide lies in its rapid inhibition when small additives are introduced into the reactive system of substances with high affinity for hydrogen atoms. Such substances inhibiting the combustion of CO are halides and halo-derivatives of hydrocarbons. Their inhibitory effect due to the termination of the reaction chains when interacting with hydrogen atoms by the reaction
2.4. Hydrocarbon burning
An examination of the combustion processes of hydrogen and carbon monoxide shows the complexity of the combustion reaction mechanism. In the case of H 2 and CO, the reaction proceeds as a chain with the participation of many elementary stages and intermediate products. Therefore, it is natural to expect that the mechanism of combustion reactions of more complex substances — hydrocarbons — is even more complex and the effects accompanying the processes of ignition and combustion of these compounds are more diverse.
Currently available information on the nature of chemical elevations of hydrocarbons in the process of their combustion allows us to explain the observed effects with some approximation.
It was established that in hydrocarbon flames, along with already known active particles, there is a large number of intermediate products of a more complex structure. In some cases, they become sources of the emergence of new chains. The main role in the processes of ignition and combustion of hydrocarbons is played by the following types of intermediate compounds:
Korolchenko A.Ya. Combustion and explosion processes
1. Hydrocarbon radicals, which is a molecule of
levodoroda, which removed one or more hydrogen atoms. These
residues are called alkyl (CH 3 - methyl; C 2 H 5 - ethyl; C 3 H 7 - propyl and
etc.). Due to its high reactivity, free alkyls
do not exist for a long time. Present in flames as intermediate
products. When interacting with other molecules behave
as an independent structural group. Hydrocarbon Radicals
usually represented by the letter R.
2. Peroxides - compounds of general formula R-00-R ".
3. Aldehydes - Type Compounds
![]()
The simplest aldehydes are formic (formaldehyde) and acetic (acetaldehyde) ![]() . These substances are all
. These substances are all
are present in products of incomplete combustion of hydrocarbons.
Chain formation during the combustion of hydrocarbons can be any reaction in which hydrocarbon radicals are formed. This can be a decomposition reaction of an ethane molecule with the formation of two free methyl groups:
![]()
or the reaction of a hydrocarbon with oxygen:
![]()
The continuation of chains occurs as a result of formation reactions | peroxide or hydroperoxide:
![]()
Chaining branching is carried out during the decomposition of hydroperoxide:
Chapter 2. Chemical processes during combustion
The given sequence of reactions leads to a gradual increase in the concentration of peroxide compounds in the reacting system.
Simultaneously with the accumulation of peroxides, radicals and starting
there are parallel reactions:

These reactions are exothermic; when they flow a large amount of heat.
With an increase in the temperature of the reaction mixture, the role of active centers passes from one intermediate product to another in the following order: alkyl hydroperoxides, acyl hydroperoxides, formaldehydes.
Experimental studies of changes in the composition of the reaction mixture over time in the high-temperature region (600-800 ° C) show that the process of transformation of the initial hydrocarbons into final combustion products is divided into two stages: the first, occurring at a very high rate, oxidizes hydrocarbons to CO. At the second, slow, stage, the CO is oxidized to. A very important conclusion follows: many of the laws of combustion of hydrocarbons can be explained by the features of the combustion of carbon monoxide.
2.5. Carbon burning
Carbon burning proceeds according to the mechanism of a heterogeneous process, the specificity of which lies in the fact that the chemical stage cannot be considered in isolation from the process of transfer of a gaseous oxidant (oxygen of the air) from the surrounding space to the surface of a burning solid. The burning rate depends on both the chemical properties of carbon and the characteristics that determine the process of supplying oxygen to the fuel surface. Oxygen supply to the combustion zone is carried out by diffusion and therefore
Korolchenko A.Ya. Combustion and explosion processes
depends on many factors: the shape and size of the burning body, the movement of the gaseous medium, the diffusion coefficients of oxygen and the reaction products both in the space above the fuel surface and in cracks and pores contained in coal and coke in significant quantities.
To illustrate the features of heterogeneous carbon burning, we consider the behavior of a separate piece of coal placed in a furnace heated to a temperature of 900 ° C. At the initial moment, the combustion of coal will occur at the expense of oxygen located near its surface. After it is used up, a layer of combustion products forms around the heated surface. The burning rate will decrease, and the process could cease if there were no oxygen coming from more distant areas of the gas space.
This flow occurs through diffusion, and the burning rate will be determined by the diffusion flux. The diffusion intensity largely depends on the intensity and nature of the movement of the gas medium near the burning surface. The rate of chemical reaction is mainly determined by temperature. Heterogeneous reactions, as well as homogeneous, obey the Arre-nius law.
At high temperatures, the oxidation reaction of carbon proceeds very quickly, and the total speed of the process will be limited by the diffusion of oxygen to the surface.
Thus, the process of burning carbon consists of two processes of different nature: the process of transporting air oxygen from the gas space to the place of its consumption and the process of its chemical interaction with the surface of solid carbon. Both of these processes are interrelated, but each has its own laws. The most important of these processes is the process of oxygen consumption, which is characterized by a variety of chemical reactions.
The mechanism of the complex reaction of combining oxygen with carbon consists in the simultaneous formation of two oxides of CO and C0 2 through an intermediate physicochemical complex of the type C X 0 Y, which is then split into CO and. The ratio between these oxides depends on the burning conditions. Accordingly, the equation for the reaction of burning carbon can be written as follows:
Chapter 2. Chemical processes during combustion
Then a homogeneous reaction of burning carbon monoxide proceeds:
the mechanism of which is discussed in section 2.3.
This reaction can take place near the carbon surface, soand inside the coal mass, in its pores and cracks.
Another reaction is a heterogeneous reaction between hot carbon and carbon dioxide:
![]()
It flows at a noticeable speed in places where there is a lack of oxygen, but where the temperature of carbon is high enough.
The combination of the reactions described determines the composition of the products of combustion of carbon.
EMERGENCE OF COMBUSTION PROCESSES
| R |
irrigation in flammable mixtures may occur as a result of their self-ignition, ignition by an external source or spontaneous combustion. If the processes of self-ignition and ignition are characteristic of substances that are in a gaseous, liquid or solid state, then spontaneous combustion is characteristic of solid materials (especially those in finely divided state) or high-boiling liquids distributed on materials with a developed surface.
3.1. Self-ignition. Stationary theory
A fire is an uncontrolled burning that develops in time and space, dangerous to people and causing material damage.
Fire hazards to humans are open fire, sparks, fever, toxic products of combustion, smoke, reduced oxygen, collapse of buildings or installations.
Combustion is a rapidly proceeding physico-chemical reaction, accompanied by the release of heat and smoke, the appearance of a flame or smoldering. Under normal conditions, combustion is the process of oxidizing or combining a combustible substance with atmospheric oxygen. However, some substances (for example, compressed acetylene, nitrogen chloride, ozone) can explode without oxygen to form heat and flame. Consequently, combustion can result from reactions not only of the compound, but also of decomposition. It is also known that hydrogen and many metals can burn in the atmosphere of chlorine, copper in sulfur vapor, magnesium in carbon dioxide, etc.
The most dangerous combustion that occurs during the oxidation of a combustible substance with oxygen in the air. At the same time, it is necessary to have an ignition source capable of supplying the required amount of energy to the fuel system. The most common sources of ignition are: sparks that appear when electrical equipment malfunctions, striking metal bodies, welding, forging works; heat generated by friction; technological heating devices; fire apparatuses; heat of adiabatic compression; spark discharge of static electricity; overheating of electrical contacts; chemical reactions proceeding with the release of heat.
The heating temperature of these sources is different. So, the spark that occurs when a metal body is struck can have a temperature of up to 1900 ° C, the flame of a match is about. 800 ° C, the leading drum of the belt conveyor during slippage is up to 600 ° C, and in the heat of the electric discharge the temperature reaches 10,000 ° C, with almost instantaneous chemical reactions.
Burning may be complete and incomplete. With complete combustion, occurring with an excess of oxygen, the reaction products are carbon dioxide, water, nitrogen, sulfur dioxide. Incomplete combustion occurs with a lack of oxygen, the combustion products in this case are toxic and combustible substances - carbon monoxide, alcohols, ketones, aldehydes, etc. A certain amount of air is necessary for the complete combustion of the combustible substance: 1 kg of wood - 4.18, peat - 5 , 8, propane - 23.8 m3.
The combustion process can be imagined as follows. A cold combustible medium with the introduction of a heat impulse is heated, there is an intensive oxidation of the combustible medium with oxygen and additional heat release. This, in turn, leads to heating of the adjacent layer of combustible material, in which an intense chemical reaction also takes place. With such a layer-by-layer combustion of a combustible substance, the combustion zone moves; The speed of this movement determines the intensity of the combustion process and is its most important characteristic. The process of layer-by-layer heating, oxidation, and combustion continues until the entire volume of combustible material is exhausted.
The narrow zone in which the substance is heated and the chemical reaction takes place is called the flame front.
Combustible systems can be chemically homogeneous and heterogeneous. Chemically homogeneous systems are mixtures of combustible gases, vapors or dusts with air, in which combustible matter and air are uniformly mixed. The burning of such systems is called homogeneous. In chemically inhomogeneous systems, combustible matter and air are not mixed and have an interface. These are often solid combustible materials and their combustion is called heterogeneous.
The total time of combustion of the combustible mixture Tg is the sum of the time required for the contact between the combustible substance and oxygen τ к to occur, and the time during which the chemical itself takes place, the oxidation reaction τ x
Depending on the ratio of these two terms, diffusion and kinetic combustion are distinguished. When burning solid combustible substances, the time required for oxygen to penetrate (diffuse) to the surface of the substance is much longer than the chemical reaction time; therefore, the total burning rate is completely determined by the rate of diffusion of oxygen to the combustible substance. The burning of such substances is most often found on fires and is called diffusive. Combustion, the rate of which is determined by the rate of chemical reaction, is called kinetic. This type of combustion is characteristic of homogeneous combustible systems.
Distinguish calorimetric, theoretical and actual temperature of burning.
Calorimetric temperature of combustion refers to the temperature to which products of complete combustion are heated, if all the heat released is spent on heating them, the amount of air is theoretically necessary, the substances are completely burned and the initial temperature is 0 ° С. Heat losses are assumed to be zero. If the initial temperature of the fuel and air is 0 ° C, then the calorimetric temperature of combustion
![]()
where Qn is the net calorific value of the combustible substance, kcal / kg; V is the volume of combustion products, m3 / kg; c is the average volumetric heat capacity of the combustion products, kcal / m3 · deg.
Consequently, the calorimetric burning temperature depends only on the properties of the combustible substance and does not depend on its quantity. The theoretical combustion temperature takes into account the heat loss during combustion to dissociate. Calorimetric burning temperature is highest for a combustible substance and is used for qualitative assessment. In reality, when burning, there are always heat losses due to radiation, heating of excess air and the environment.
The actual burning temperature is the fire temperature. Distinguish the temperature of the internal and external fire. The temperature of the external fire is the temperature of the flame, and the internal temperature is the temperature of the smoke in the room. Actual temperatures developing in case of fire due to heat loss to the environment, heating of combustion products and structures
always less than theoretical by 30 ... 50%. For example, the theoretical burning temperature of gasoline is 1730 ° C, and the actual temperature is 1400 ° C.
A mixture of combustible vapors and gases with an oxidizing agent can burn only with a certain content of fuel in it.
The lowest concentration of combustible gas at which combustion is already possible is called the lower concentration limit of ignition (LEL). The highest concentration at which combustion is still possible is called the upper concentration limit of ignition (AIPW). The region of concentration that lies within these boundaries is called the region of ignition. Ignition is a fire (the beginning of burning), accompanied by the appearance of a flame. This is a steady long burning, which does not stop even after the ignition source is removed. The values \u200b\u200bof the lower and upper limits of ignition depend on the properties of gas, vapor and dust of air mixtures, the content of inert components in the combustible mixture. The addition of inert gases to the combustible mixture narrows the area of \u200b\u200bignition and eventually makes it non-combustible. Significantly narrow the limits of ignition, some impurities that slow down the combustion reaction. The most active of these are halogenated hydrocarbons. Both marked properties are used to stop burning. Lowering the pressure of the mixture below atmospheric pressure also narrows the area of \u200b\u200bignition, and at a certain pressure the mixture becomes incombustible. Increasing the pressure of the combustible mixture expands the area of \u200b\u200bignition, but, as a rule, slightly. Increasing the temperature of the combustible mixture expands the area of \u200b\u200bignition. The concentration of ignition is also affected by the power of the ignition source.
There are not only concentration, but also temperature limits of ignition.
Temperature limits for the ignition of vapors in air are those temperatures of a combustible substance at which its saturated vapors form concentrations corresponding to the lower or upper concentration limit of ignition. The ignition temperature is the lowest temperature at which a substance ignites or begins to smolder and continues to burn or smolder after removing the source of ignition. The ignition temperature characterizes the ability of a substance to self-combustion. If the ignition temperature of a substance is absent, then it is referred to as slow-burning or non-combustible.
The acceleration of the oxidation reaction under the action of temperature leads to self-ignition. Unlike the ignition process, in which only a limited part of the volume - the surface - ignites, self-ignition occurs in the entire volume of the substance. The self-ignition temperature is understood as the lowest temperature, to which the substance must be heated, so that it will ignite as a result of further auto-oxidation. Self-ignition is possible only if the amount of heat released during the oxidation process exceeds the heat release to the environment.
Auto-ignition temperature is not constant for a substance, since it largely depends on the conditions of its determination. For obtaining comparative data, the test apparatus and the method for determining the self-ignition temperature of gases and vapors are standardized (GOST 13920-68). The lowest temperature determined by the standard method, to which the mixture of gases and vapors with air must be uniformly heated in order for it to ignite without introducing an external ignition source into it, is called the standard auto-ignition temperature.
A type of spontaneous ignition is spontaneous combustion, i.e., combustion as a result of self-heating without the influence of an ignition source. The difference between spontaneous ignition and spontaneous combustion is in the magnitude of the temperature. Spontaneous combustion occurs at ambient temperature, and for self-ignition it is necessary to heat the substance from the outside.


 What will happen if you do not transmit meter readings
What will happen if you do not transmit meter readings Tyumenenergosbyt personal account Tyumen
Tyumenenergosbyt personal account Tyumen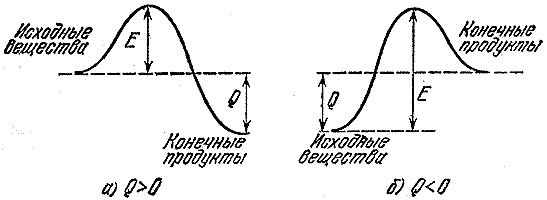 Characteristics of the combustion process
Characteristics of the combustion process